- Introduction
- Anatomy
- Physiology
- Symptoms and Signs of Esophageal Diseases
- Investigations Used in the Diagnosis of Esophageal Disease
- Nonreflux-Induced Esophagitis
- Disorders of the Oropharyngeal Phase of Deglutition
- The Esophagus as a Cause of Angina-Like Chest Pain
- Esophageal Neoplasms
- Miscellaneous Disorders of the Esophagus
1. Introduction
The esophagus is a hollow muscular organ whose primary function is to propel into the stomach the food or fluid bolus that it receives from the pharynx. Symptoms of esophageal disease are among the most commonly encountered in gastroenterology. Fortunately, most symptoms are due to benign disease that can be easily remedied. The physician must be on the lookout, however, for the more serious disorders, which can present with a similar spectrum of symptoms. This chapter will focus on the pathophysiology, diagnosis and management of the more common esophageal disorders. Rare diseases involving the esophagus will be dealt with only briefly.
2. Anatomy
2.1. Muscular Anatomy
The esophagus is a hollow muscular tube closed proximally by the upper esophageal sphincter (UES) and distally by the lower esophageal sphincter (LES). The UES consists predominantly of the cricopharyngeus and the caudal fibers of the inferior pharyngeal constrictor muscles. The UES forms a transverse slit at the C5–C6 vertebral level due to surrounding bony structures and cartilage. In the proximal one-quarter to one-third of the esophagus, the muscle is striated. There is then a transition zone of variable length where there is a mixture of both smooth and striated muscle. The distal one-half to one-third of the esophageal body and LES are composed of smooth muscle. The LES is located at the junction between the esophagus and stomach, usually localized at or just below the diaphragmatic hiatus. With careful dissection, the LES can be identified as an area of thickened circular smooth muscle consisting of two components, namely, semi-circular “clasp” fibers on the lesser curvature, and “sling-like” muscle bundles on the greater curvature that merge with the long oblique gastric muscle fibers.
2.2 Innervation
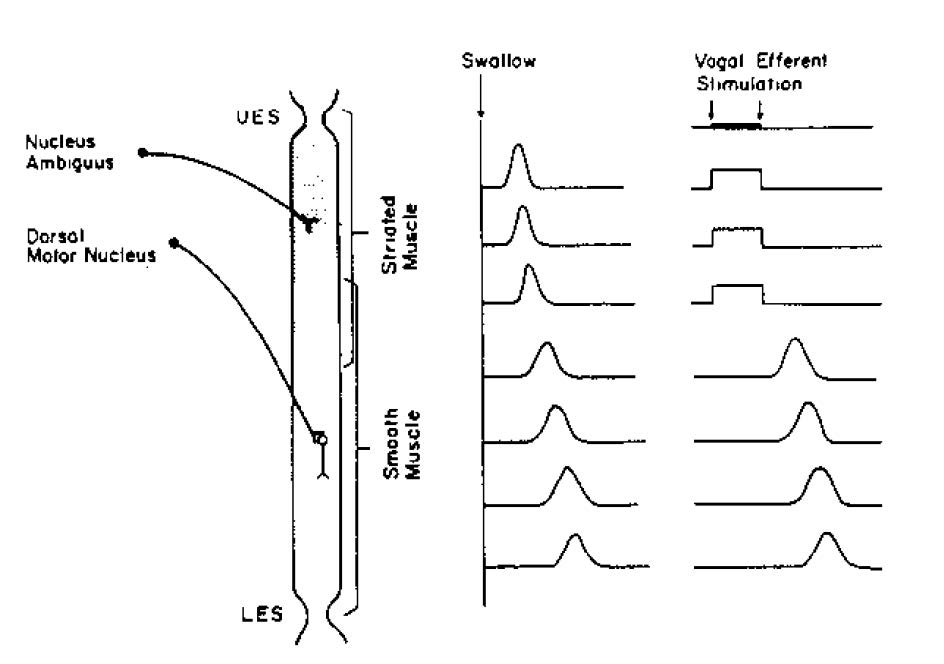
The motor innervation of the esophagus is via the vagus nerves. The cell bodies of the vagal efferent fibers innervating the UES and the proximal striated-muscle esophagus arise in the nucleus ambiguus, whereas fibers destined for the distal smooth-muscle segment and the LES originate in the dorsal motor nucleus. The esophagus and LES also receive sympathetic nerve supply (both motor and sensory) arising from spinal segments T1–T10. Sensory innervation is also carried via the vagus and consists of bipolar nerves that have their cell bodies in the nodose ganglion and project from there to the brainstem.
2.3. Blood Supply
Arterial blood supply to the UES and cervical esophagus is via branches of the inferior thyroid artery. Most of the thoracic esophagus is supplied by paired aortic esophageal arteries or terminal branches of bronchial arteries. The LES and the most distal segment of the esophagus are supplied by the left gastric artery and by a branch of the left phrenic artery. Venous drainage is via an extensive submucosal plexus that drains into the superior vena cava from the proximal esophagus and into the azygous system from the mid-esophagus. In the distal esophagus, collaterals from the left gastric vein (a branch of the portal vein) and the azygos interconnect in the submucosa. This connection between the portal and systemic venous systems is clinically important; when there is portal hypertension, variceal dilation can occur in this area. These submucosal esophageal varices can be the source of major gastrointestinal hemorrhage.
2.4. Lymphatic Drainage
In the proximal third of the esophagus, lymphatics drain into the deep cervical lymph nodes, whereas in the middle third, drainage is into the superior and posterior mediastinal nodes. The distal-third lymphatics follow the left gastric artery to the gastric and celiac lymph nodes. There is considerable interconnection among these three drainage regions.
2.5. Histology
The wall of the esophagus consists of mucosa, submucosa and muscularis propria. Unlike other areas of the gut, it does not have a distinct serosal covering, but is covered by a thin layer of loose connective tissue. The mucosa consists of stratified squamous epithelium in all regions of the esophagus except the LES, where both squamous and columnar epithelium may coexist. Beneath the epithelium are the lamina propria and the longitudinally oriented muscularis mucosa. The submucosa contains connective tissue as well as lymphocytes, plasma cells and nerve cells (Meissner’s plexus). The muscularis propria consists of an inner circular and an outer longitudinal muscle layer. The circular muscle layer provides the sequential peristaltic contraction that propels the food bolus toward the stomach. Between the circular and longitudinal muscle layers lies another nerve plexus called the myenteric or Auerbach’s plexus, which mediates much of the intrinsic nervous control of esophageal motor function.
3. Physiology
The major function of the esophagus is to propel swallowed food or fluid into the stomach. This is carried out by sequential or “peristaltic” contraction of the esophageal body in concert with appropriately timed relaxation of the upper and lower esophageal sphincters. The esophagus also clears any refluxed gastric contents back into the stomach and takes part in such reflex activities as vomiting and belching.
3.1. Deglutition: Primary Peristalsis
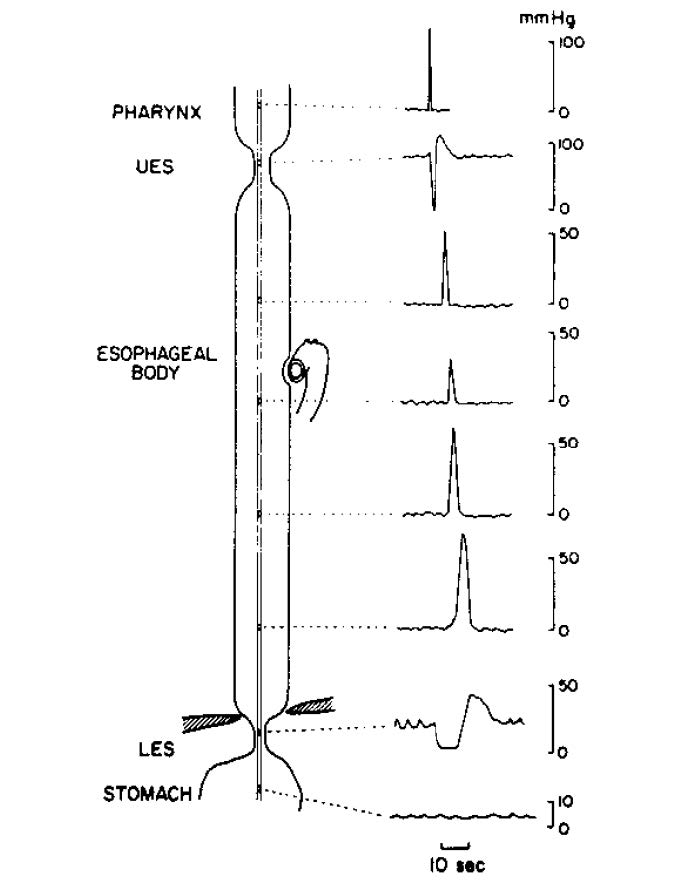
The act of deglutition is a complex reflex activity. The initial phase is under voluntary control. Food is chewed, mixed with saliva and formed into an appropriately sized bolus before being thrust to the posterior pharynx by the tongue. Once the bolus reaches the posterior pharynx, receptors are activated that initiate the involuntary phase of deglutition. This involves the carefully sequenced contraction of myriad head and neck muscles. The food bolus is rapidly engulfed and pushed toward the esophagus by the pharyngeal constrictor muscles. Simultaneously there is activation of muscles that lift the palate and close off and elevate the larynx in order to prevent misdirection of the bolus. Almost immediately upon activation of this reflex, the UES opens just long enough to allow the food bolus to pass through; it then rapidly shuts to prevent retrograde passage of the bolus. The oropharyngeal phase is thus completed and the esophageal phase takes over. This involves two major phenomena: (1) the sequential contraction of the circular muscle of the esophageal body, which results in a contractile wave that migrates toward the stomach; and (2) the relaxation and opening of the LES, which allows the bolus to pass. The peristaltic sequence and associated UES and LES relaxation induced by swallowing are termed primary peristalsis. These can be assessed manometrically using an intraluminal tube to measure pressures. The typical sequence seen during primary peristalsis is depicted in Figure 1. Secondary peristalsis refers to a peristaltic sequence that occurs in response to distention of the esophagus. This is a localized peristaltic wave that usually begins just above the area of distention. It is associated with LES relaxation, but not with UES relaxation or deglutition.
3.2. Upper Esophageal Sphincter Function
The UES serves as a pressure barrier to prevent retrograde flow of esophageal contents and the entry of air into the esophagus during inspiration. This high pressure zone is created by tonic contraction of the UES muscles, which is produced by tonic neuronal discharge of vagal lower motor neurons. With deglutition this neuronal discharge ceases temporarily and permits relaxation of the UES. UES opening will not occur with relaxation of the muscles alone; it requires elevation and anterior displacement of the larynx, which is mediated by contraction of the suprahyoid muscles. Relaxation lasts for only one second and is followed by a post- relaxation contraction (Figure 1).
3.3. Esophageal Body Peristalsis
There is a fundamental difference in the control mechanisms of peristalsis between the upper (striated-muscle) esophagus and the lower (smooth-muscle) esophagus. In the striated-muscle segment, peristalsis is produced by sequential firing of vagal lower motor neurons so that upper segments contract first and more aboral segments subsequently. In the smooth-muscle segment, the vagal preganglionic efferent fibers have some role in the aboral sequencing of contraction, but intrinsic neurons are also capable of evoking peristalsis independently of the extrinsic nervous system. Transection of vagal motor fibers to the esophagus in experimental animals will abolish primary peristalsis throughout the esophagus; however, in this setting, distention-induced or secondary peristalsis will be maintained in the smooth-muscle but not in the striated-muscle segment. Furthermore, if vagal efferent fibers are stimulated electrically (Figure 2), a simultaneous contraction will be produced in the striated-muscle esophagus that begins with the onset of the electrical stimulus, lasts throughout the stimulus, and ends abruptly when the stimulus is terminated. In the smooth-muscle esophagus, however, the response to vagal efferent nerve stimulation is quite different, in that the onset of contractions is delayed relative to the onset of the stimulus. The latency to onset of the contraction increases in the more distal segments of the esophagus (i.e., the evoked contractions are peristaltic).
This experimental observation indicates that intrinsic neuromuscular mechanisms exist and can mediate peristalsis on their own. Further evidence for this mechanism is found in studies where strips of esophageal circular smooth muscle are stimulated electrically in vitro. The latency to contraction after stimulation is shortest in the strips taken from the proximal smooth-muscle segment and increases progressively in the more distal strips.
This latency gradient of contraction is clearly important in the production of esophageal peristalsis. Although the exact mechanisms are unclear, initial or deglutitive inhibition is important. With primary or secondary peristalsis, a wave of neurally mediated inhibition initially spreads rapidly down the esophagus. This is caused by the release of the inhibitory neurotransmitter nitric oxide, which produces hyperpolarization (inhibition) of the circular smooth muscle. It is only after recovery from the initial hyperpolarization that esophageal muscle contraction (which is mediated primarily by cholinergic neurons) can occur. Thus, the duration of this initial inhibition is important with respect to the differential timing of the subsequent contraction. Derangements of the mechanisms behind this latency gradient lead to nonperistaltic contractions and dysphagia. Such derangements could result from problems with either the intrinsic neural mechanisms (enteric nervous system) or the central neuronal sequencing.
3.4. Lower Esophageal Sphincter Function
The LES is an intraluminal high-pressure zone caused by tonic contraction of a region of physiologically distinct circular smooth muscle at the junction of the esophagus and stomach. This results in a pressure barrier that separates the esophagus from the stomach and serves to prevent reflux of gastric contents up into the esophagus. In normal individuals, resting LES pressure averages between 10 and 35 mmHg above intragastric pressure. Patients with very feeble resting LES pressure are prone to develop gastroesophageal reflux disease (GERD). Unlike that of the UES, the resting tone of the LES is primarily due to myogenic factors that result in tonic contraction of the sphincter. Extrinsic innervation as well as circulating hormones can modify the resting tone; however, the muscle fibers themselves have inherent properties that result in their being tonically contracted.
At the time of deglutition or when the esophagus is distended, the LES promptly relaxes. Swallow-induced LES relaxation is mediated by vagal efferent fibers that synapse on inhibitory neurons of the myenteric plexus. The predominant inhibitory neurotransmitter released from these intrinsic neurons is nitric oxide. LES relaxation usually lasts about five to seven seconds, and is sufficient to abolish the gastroesophageal pressure barrier. This permits the food bolus to pass unimpeded from the esophagus to the stomach. The LES also relaxes to permit belching or vomiting. Inadequate LES relaxation is seen in achalasia and results in dysphagia.
4. Symptoms and Signs of Esophageal Diseases
4.1.Symptoms
4.1. Dysphagia
The sensation of food sticking during swallowing is a manifestation of impaired transit of food through the mouth, pharynx or esophagus. It is important to differentiate oropharyngeal (“transfer”) dysphagia from esophageal dysphagia. If the patient has problems getting the bolus out of the mouth, then one can be certain of an oropharyngeal cause; if the food sticks retrosternally, an esophageal cause is indicated. Some patients, however, will sense food sticking at the level of the suprasternal notch when the actual obstruction is the distal esophagus. Thus, it can be difficult to determine the site of the problem when patients refer their dysphagia to the suprasternal notch or throat area. With these patients it is important to elicit any ancillary symptoms of oropharyngeal-type dysphagia, such as choking or nasal regurgitation. It may also be helpful to observe the patient swallowing in an attempt to determine the timing of the symptom; with esophageal dysphagia referred to the suprasternal notch, the sensation of dysphagia onsets several seconds after swallowing begins.
The history can also be used to help differentiate structural from functional (i.e., motility disorders) causes of dysphagia. Dysphagia that is episodic and occurs with both liquids and solids from the outset suggests a motor disorder, whereas when the dysphagia is initially for solids such as meat and bread, and then progresses with time to semisolids and liquids, one should suspect a structural cause (e.g., stricture). If such a progression is rapid and associated with significant weight loss, a malignant stricture is suspected. Associated symptoms help determine the etiology of dysphagia. For instance, a reflux-induced stricture should be suspected if the dysphagia is associated with heartburn or regurgitation, esophageal cancer if there is associated mid-back pain and weight loss, a motor disorder such as diffuse esophageal spasm if there is angina-like chest pain, and a “scleroderma esophagus” if there is arthralgia, skin changes or Raynaud’s phenomenon.
4.1.2. Odynophagia
This refers to the sensation of pain on swallowing. Local inflammation or neoplasia in the mouth and pharynx can produce such pain. When the pain is retrosternal, one should suspect nonreflux-induced forms of esophagitis, such as infection, radiation or pill-induced (chemical) injury. Less commonly it occurs with esophageal cancer, a deep esophageal ulcer (e.g., Barrett’s ulcer) or esophageal motor disorders.
4.1.3. Heartburn or Pyrosis
The sensation here is one of retrosternal burning. Typically it begins in the low retrosternal area and radiates up to the throat. It may be precipitated by bending over or lying down, and usually begins shortly after consuming certain foods or beverages. It is often associated with
regurgitation of acidic material into the back of the throat. “Heartburn” with these features indicates gastroesophageal reflux. This very common symptom has been experienced at one time or another by over one-third of the population and therefore does not necessarily indicate serious disease. Many patients will complain of “heartburn,” but this should not be taken at face value: this term is used by some patients to describe unrelated symptomatology. It is therefore important to have patients describe exactly what they mean by the term heartburn.
4.1.4. Regurgitation
This refers to the spontaneous appearance of food or fluid in the back of the throat or in the mouth. Some patients describe this symptom as “vomiting”; therefore it is important to determine whether there is associated nausea, retching, etc., when patients present with “vomiting.” The taste and consistency of the regurgitated material is an important historical detail. Regurgitation of acidic or bile-stained fluid indicates gastroesophageal reflux. Regurgitation of undigested food or stagnant fluid devoid of an acidic taste indicates an esophageal transport problem (e.g., achalasia). (With achlorhydria, such as occurs with pernicious anemia, gastric contents also lack acid.) In motor disorders and mechanical obstruction of the esophagus, food may become stuck and then rather quickly will be regurgitated if it does not pass through into the stomach. Some patients regurgitate food back into their mouths after a meal only to chew and swallow it all over again. This is called rumi nation and, although a rarity in humans, it is a normal physiological event in certain animals.
4.1.5. Nonheartburn Chest Pain
This may also be an indication of esophageal disease. Chest pain, and in particular mid- dorsal pain, is seen in advanced esophageal cancer. The most common type of nonheartburn esophageal chest pain, however, is a pain that is qualitatively similar to the pain of ischemic heart disease (so-called “noncardiac chest pain”). This pain can be squeezing or crushing and can radiate into the jaw or arms. Unlike ischemic heart pain, angina-like chest pain of esophageal origin is not predictably elicited by exertion and often occurs spontaneously, in relationship to meals or in the middle of the night. It may be associated with other more typical esophageal symptoms. Clearly, patients with this type of pain need to have ischemic heart disease excluded. Once this is done, many will be found to have either gastroesophageal reflux or some form of esophageal motor or sensory disorder.
4.1.6. Waterbrash
The sudden appearance of copious amounts of saliva in the mouth must be differentiated from regurgitation of fluid. With waterbrash, acid reflux into the esophagus stimulates hypersalivation via a (cholinergic) neural reflex.
4.1.7. Bleeding
This may be a symptom of certain esophageal diseases. Mucosal laceration in the region of the gastroesophageal junction (M allory-Weiss tear), as a consequence of retching or vomiting, is a common cause of upper gastrointestinal tract bleeding. Esophageal varices can cause massive hematemesis and melena. Deep esophageal ulcers may also bleed massively, but this is uncommon. Usually the bleeding from ulcerative lesions of the esophagus or esophageal cancer is occult. When the patient does present with hematemesis or melena from esophagitis, the rate of bleeding is usually slow; therefore, significant hemodynamic compromise is uncommon.
4.1.8. Respiratory/Laryngeal Symptoms
These may be a manifestation of esophageal disease or oropharyngeal swallowing disorders. Aspiration at the time of swallowing will cause coughing, choking and eventual hoarseness. In addition, patients with motor disorders or gastroesophageal reflux disease (GERD) may regurgitate esophageal or gastric contents up into the larynx and subsequently aspirate. These patients may present with pneumonia, chronic cough, wheezing, hoarseness or laryngitis. Gastroesophageal reflux might also trigger coughing and wheezing via a vagovagal reflex.
4.2. Signs
It is uncommon for esophageal disease to be associated with specific physical findings. Signs of weight loss and malnutrition can be found if the esophageal problem is so severe that adequate caloric intake is not maintained. There may be signs of metastatic disease (e.g., hepatomegaly, supraclavicular lymphadenopathy) in esophageal cancer. Patients with GERD rarely have respiratory tract signs such as wheezing, hoarseness or lung consolidation. It is important to look for signs of connective tissue disease (especially scleroderma) in patients with reflux symptoms or dysphagia.
The physical examination is more often helpful in patients with oropharyngeal dysphagia. Careful examination of the head and neck for structural and neurologic abnormalities is mandatory. It is also important to look for more generalized neurologic or connective tissue abnormalities. Observing the patient swallow is also useful when oropharyngeal dysphagia is present.
5. Investigations Used in the Diagnosis of Esophageal Disease
A number of tests are available to facilitate the diagnosis of patients with suspected esophageal disease. The choice of which diagnostic test to use depends on the patient presentation and the question(s) to be answered.
5.1. Barium X-ray
This most commonly used method of investigating the esophagus evaluates both structural lesions and motor disorders. It is most useful in evaluating patients with dysphagia. Proper communication between physician and radiologist is vital. Videotaping the barium swallow (“video-fluoroscopy swallowing study”) allows for playback and slow-motion review. This is very helpful in assessing the rapid events of the oropharyngeal phase of swallowing. Use of marshmallows, barium-coated cookies and different consistencies of barium further assesses swallowing disorders, as delays in transport may not be apparent with simple liquid barium. The disadvantage of barium x-rays is that they are relatively insensitive in detecting mucosal disease. If a patient is suspected of having an esophageal perforation, a water soluble contrast agent (Gastrograffin) should be used in place of barium.
5.2. Endoscopy with Mucosal Biopsy and Brush Cytology
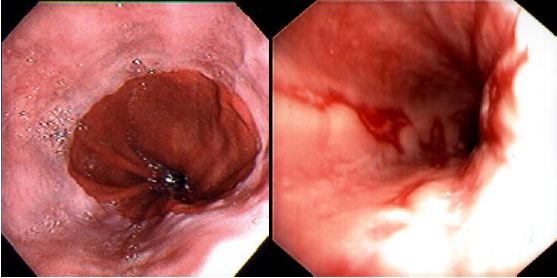
Fiberoptic endoscopy directly visualizes the esophageal mucosa as well as other areas of the upper gastrointestinal tract. Its direct view is superior to barium x-rays for assessing mucosal disease of the esophagus. Furthermore, pinch biopsies and/or brush cytology of specific lesions are easily obtained through the endoscope. Microscopic evidence of esophagitis may be found even when the mucosa looks grossly normal. Endoscopy is the single most useful test in the evaluation of patients with reflux symptoms, as it permits one to establish the presence or absence of esophagitis (Figure 3) or Barrett’s esophagus (Section 7.3). Endoscopy gives little reliable information regarding esophageal function.
5.3. Endoscopic Ultrasound
This technique combines ultrasonography with endoscopy by placing an ultrasound transducer at the end of a video endoscope. It is particularly useful in staging esophageal cancer in that it is the most sensitive imaging technique for determining the depth of invasion through the esophageal wall and involvement of region lymph nodes.
5.4. Esophageal Manometry
This involves recording intraluminal pressures at multiple sites along the esophagus (Figure 1). The most commonly used method involves a perfused multilumen catheter bundle with side holes at 5 cm intervals. Each catheter is connected to a pressure transducer, which in turn is attached to a physiograph. LES pressure and swallow-induced LES relaxation are measured, as are pressure responses to swallowing at several esophageal sites. Pharyngeal peristalsis and UES function can also be measured. Esophageal manometry is the “gold standard” in the assessment of esophageal motor disorders. Motor dysfunction, however, may be intermittent and therefore not detected at the time of the study. Manometry may be combined with provocative tests (acid perfusion, balloon distention and/or pharmacological stimulation of the esophagus with bethanechol or edrophonium) in an attempt to evoke abnormal contractions and reproduce the patient’s chest pain (Section 11). In recent years, the introduction of “high resolution” manometry has allowed for more detailed recording and analysis of esophageal motor function. Using multiple pressure sensors spaced at 1 cm intervals, the pressure profile from pharynx to stomach can be assessed simultaneously. Sophisticated software converts the data to contour plots using different colours to depict pressure variations, thereby facilitating detection of motor disorders. The technique can be combined with simultaneous intraluminal impedance recording, so that bolus transit can be simultaneously measured and correlated with motor function. This powerful methodology enhances the detection of esophageal motor disorders, but is quite expensive.
5.5. Ambulatory Esophageal pH Monitoring
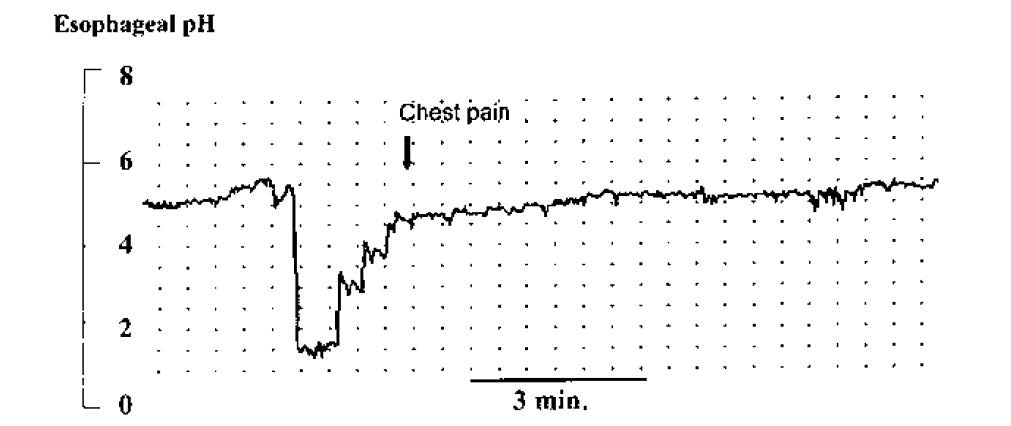
This is performed using a pH electrode passed via the nose into the distal esophagus, which continuously records intraluminal pH over a 24-hour period. Acid reflux events can be identified by an abrupt drop in pH to < 4. The results of this test are compared to a healthy control population to determine whether an abnormal degree of gastroesophageal acid reflux is present. The test is most useful, however, in determining whether atypical symptoms coincide with acid reflux events (Figure 4), and in objectively assessing the response to therapy in patients with refractory symptoms. Recently, wireless pH electrodes, which are clipped to the distal esophageal mucosa endoscopically, have been introduced. These are better tolerated and allow for longer recording intervals (e.g. 48-72 hours), which increases diagnostic yield. In addition, combined pH and impedance recording catheters are being used at some centres, and are useful in detecting non-acid or weakly acidic reflux events that may be responsible for refractory symptoms in a small subset of patients.
6. Anatomic Variants
6.1. Congenital Anomalies
Embryologically the gastrointestinal and respiratory tracts start out as a single tube; however, by the second month of gestation they have completely divided. Problems with this process lead to various congenital anomalies, the most common being tracheoesophageal fistula with esophageal atresia. In 85–90% of cases, the proximal esophagus ends in a blind pouch while the distal esophagus consists of a blind pouch in continuity with the stomach. Neonates with this abnormality develop immediate aspiration with feeding. There is no air in the bowel on x-ray films of the abdomen, contrary to what is observed in those with fistulas involving the distal esophagus. In 1–2% of cases there is an “H-type” fistula with atresia. The patient presents with repeated pulmonary infections and abdominal distention. The latter is caused by air getting into the gastrointestinal tract via the fistula when the infant cries. Because the H-type fistula may be very small, the condition may go unnoticed until adulthood, when it is detected during the investigation of recurrent pulmonary infections. Some of these fistulas may close spontaneously but produce paraesophageal inflammation and ultimately localized esophageal stricture formation.
Treatment of esophageal fistulas (with or without atresia) is surgical. The prognosis is now quite good and mortality is usually related to coexistent congenital malformations. It is important to remember that many of these patients will have gastroesophageal reflux as well as abnormal esophageal peristalsis following surgery, which may cause significant long-term problems.
Congenital esophageal stenosis is a rare anomaly that is also probably related to abnormal differentiation of the gastrointestinal and respiratory tracts, as resected specimens have been found to have pulmonary epithelium and/or bronchial remnants. Sequestered pulmonary remnants with connections to the esophagus but not associated with stenosis have also been described.
6.2. Hiatus Hernia
The majority of hiatus hernias are acquired. Rarely, a hiatus hernia can be caused by a congenitally short esophagus. Hiatus hernias can be divided into two types: (1) sliding and (2) paraesophageal (Figures 5 and 6, respectively). A sliding hiatus hernia refers to the condition where a circumferential cuff of cardia and proximal stomach migrates up through the diaphragmatic hiatus and into the thorax. This may reduce and reform spontaneously. These hernias are very common and increase in incidence with advancing age. Generally they are of no clinical significance, despite the fact that many patients and physicians persist in attributing a wide variety of symptoms to them. Large hiatus hernias may be associated with iron deficiency anemia that is presumably caused by recurrent superficial ischemic ulcerations at the site where the diaphragm exerts pressure on the herniated stomach (“Cameron’s” ulcers). If no other source of GI blood loss is discovered after thorough investigation, and patients continue to be iron- deficient despite supplementation and antiulcer treatment, surgical correction of the hernia should be performed.
The etiology of the sliding hiatus hernia is obscure. Certainly there is laxity and dilation of the diaphragmatic hiatus and associated laxity of the phrenoesophageal ligament; however, these may well be secondary and not primary pathophysiologic factors. In some cases, persistent gastroesophageal reflux may result in inflammation and consequent esophageal shortening, which in turn leads to the development of a hiatus hernia.
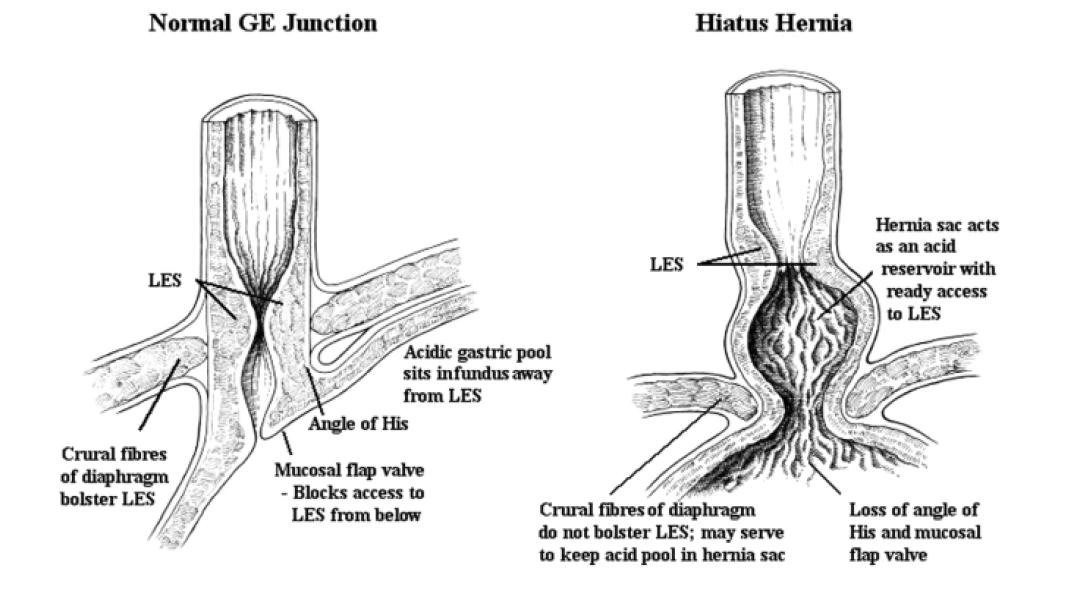
A sliding hiatus hernia is often seen in association with GERD; the precise role of the hernia in the pathogenesis of the reflux remains uncertain. The majority of people with hiatus hernias do not have significant reflux disease, and occasionally patients with severe reflux esophagitis will not have a hiatus hernia. It appears that a hiatus hernia may contribute to gastroesophageal reflux (see Figure 5), but it is most unlikely that this is the prime etiologic factor. A hiatus hernia may contribute to GERD by providing a reservoir of gastric acid that has ready access to the distal esophagus whenever the LES relaxes.
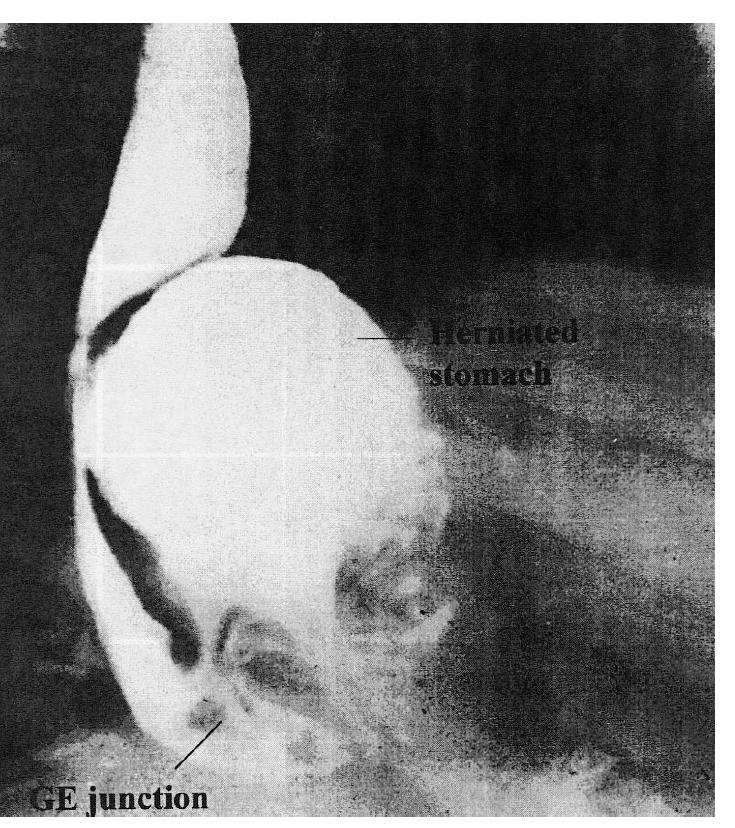
Paraesophageal hiatus hernias are uncommon. These consist of the fundus of the stomach migrating through the hiatus alongside the esophagus without any displacement of the gastroesophageal junction. Although these hernias may be asymptomatic, many surgeons believe that they should be treated surgically when the diagnosis is made because the herniated portion may become strangulated and infarcted. However, a recent study suggests that observation alone is a valid option. Paraesophageal hernias may also cause dysphagea by compressing the distal esophagus (Figure 6). The treatment consists of reduction of the herniated stomach into the abdomen, elimination of the hernia sac and closure of the herniated defect by reapproximating the crura. Whether or not an anti-reflux procedure (i.e. fundoplication) should be added is debatable. On occasion, both types of hiatus hernias can coexist in the same patient (mixed hiatus hernia).
7. Gastroesophageal Reflux Disease (GERD)
GERD is the most common condition to affect the esophagus, with roughly 5 million Canadians experiencing heartburn or acid regurgitation, the cardinal symptoms of GERD, at least once per week. The disease spectrum ranges from patients with heartburn and other reflux symptoms without morphologic evidence of esophagitis (the so-called endoscopy-negative reflux disease) to patients with deep ulcer, stricture or Barrett’s epithelium. Everyone has some degree of gastroesophageal reflux; it becomes pathological only when associated with troublesome symptoms or complications. Fortunately, the vast majority of patients suffering from GERD have an easily controlled disorder. At the other end of the spectrum, there are patients who develop severe damage to the esophagus. Some will develop Barrett’s metaplasia as a consequence of gastroesophageal reflux, which in turn predisposes them to adenocarcinoma.
7.1.Pathophysiology
GERD results from the reflux of gastric contents into the esophageal lumen. Early pathogenesis concepts focused on anatomic factors: reflux was considered a mechanical problem, related to the development of a hiatus hernia. We now know, however, that a hiatus hernia can occur without GERD, and conversely, GERD can occur without a hiatus hernia. Many factors are involved in the pathogenesis of GERD.
7.1.1. Barriers to Gastroesophageal Reflux
By far the most important barrier to gastroesophageal reflux is the LES. Factors such as the intra-abdominal location of the sphincter, extrinsic compression exerted by the diaphragmatic crura and the angle of His (which forms a “mucosal flap valve”) may augment this barrier but plays a less significant role than the LES itself (Figure 5). Some patients developing reflux esophagitis have feeble LES tone, but in most, resting LES pressure is normal or only slightly impaired. Gastroesophageal reflux occurs by three major mechanisms, as outlined in Figure 7.
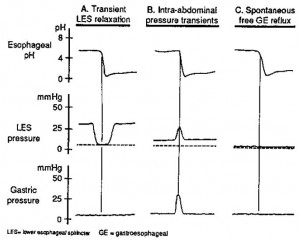
A. Transient LES relaxation refers to the sudden occurrence of LES relaxation that causes obliteration of the gastroesophageal pressure barrier and permits gastric contents to reflux up into the esophagus. The reflux event is marked by the sudden drop in esophageal pH. These transient LES relaxations are sometimes related to incomplete or failed peristalsis but may also occur in isolation.
B. Intra-abdominal pressure transients are sudden increases in intragastric pressure caused by coughing, sneezing or deep inspiration. The increased intragastric pressure overcomes the LES pressure and results in reflux.
C. Spontaneous free reflux occurs when there is very low or nonexistent LES pressure, which permits spontaneous reflux across the gastroesophageal junction. In healthy volunteers without GERD, virtually all reflux episodes are due to transient LES relaxation. In patients with reflux esophagitis, approximately two-thirds of the reflux episodes are due to transient LES relaxation. The remaining one-third are caused by either intra-abdominal pressure transients or spontaneous free gastroesophageal reflux.
Source: Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. The New England Journal of Medicine1982; 307:1547–1552. Used with permission.
7.1.2. Esophageal Clearance
Once reflux occurs, the duration of insult to the esophageal mucosa depends on the rapidity with which the esophagus clears this material. Once the initial (primary) peristaltic wave has passed, the bolus (a portion of which frequently remains) is cleared by one or two secondary peristaltic waves. The remaining small adherent acidic residue is then neutralized by saliva, which is carried down by successive swallows. Disorders of salivation or esophageal motor function will impair this clearance mechanism and predispose to the development of GERD. Patients with severe GERD may have frequent prolonged nighttime reflux episodes because during sleep, peristalsis seldom occurs and salivary flow virtually ceases. Hence the contact time of refluxed material with the esophagus is markedly increased.
7.1.3. Gastroduodenal Factors
In some patients delayed gastric emptying further predisposes to the development of GERD. Bile salts and pancreatic enzymes, if refluxed back into the stomach, can in turn reflux into the esophagus and may inflict worse damage than when gastric juice is refluxed alone. Such reflux into the stomach and then the esophagus may be significant after gastric surgery, when the pylorus is destroyed. Whenever there is increased gastric pressure or an increase in gastric contents, there is greater likelihood that reflux will occur when the sphincter barrier becomes deficient. Furthermore, distention of the proximal stomach is a potent stimulus for transient LES relaxation via a vago-vagal reflex.
7.1.4 Mucosal Resistance
The degree of damage to esophageal mucosa depends not only on the composition of the refluxed material and the amount and duration of reflux, but also on defensive factors within the mucosa itself. These include protective secretions from esophageal glands, the integrity of tight junctions between adjacent epithelial cells and esophageal blood flow. Certain patients are more susceptible to the development of actual mucosal damage, for reasons that are not clear.
7.2. Clinical Features
Most patients present with heartburn and acid regurgitation that onset after eating certain foods or following various postural maneuvers (e.g., bending over, lying flat). Frequency varies from once a week or less to daily episodes with disruption of sleep. Other presenting symptoms include waterbrash, angina-like chest pain, dysphagia and various respiratory symptoms
(hoarseness, throat discomfort, cough, wheezing). The dysphagia may be due to the development of a reflux-induced stricture, loss of compliance of the esophageal wall secondary to inflammation, or to abnormal motility induced by the refluxed acid. Odynophagia is rarely a symptom of GERD and should alert the physician to another diagnosis such as infectious esophagitis.
Reflux symptoms are common during pregnancy because of increased intra-abdominal pressures and the LES-relaxant effect of progesterone. Physical examination in patients with GERD rarely reveals associated physical signs. In severe cases with stricture formation there may be weight loss secondary to decreased caloric intake. Patients with GERD secondary to scleroderma may have the physical findings associated with this disease.
7.3. Diagnosis
In the vast majority of patients, GERD can be diagnosed from the history alone and treated without further investigation. Some specialists believe that all patients with longstanding symptomatic gastroesophageal reflux should undergo endoscopy. The argument in favour of this approach is that Barrett’s esophagus will be found in about 5-10% of patients with GERD symptoms for more than 5 years. This identifies those at increased risk for the development of adenocarcinoma (Section 7.5.2). Such an approach is of unproven benefit, however, and is almost certainly not cost-effective. Less than half the patients undergoing endoscopy for reflux symptoms will have erosive esophagitis. The remainder have non-erosive reflux disease (“NERD”). Endoscopic biopsy in these patients may detect microscopic evidence of esophagitis (hyperplasia of the basal zone layer, elongation of the papillae, inflammatory cell infiltration, dilated intercellular spaces).
In patients with atypical or multiple symptoms, or typical symptoms that don’t respond to empiric treatment, a 24-hour pH reflux study may be necessary to establish that the symptom(s) are in fact due to acid reflux (Figure 4). It is important to first rule out ischemic heart disease if the presenting symptom is angina-like chest pain. In general, patients who present with symptoms of complicated GERD (i.e., dysphagia, bleeding or respiratory symptoms) require investigation. If dysphagia is present, an upper GI endoscopy, with or without initial barium x- ray study, should be performed. It may be reasonable to forgo further testing in patients with heartburn and dysphagia that completely resolve with proton pump inhibitor therapy.
Esophageal manometry has little role to play in the routine assessment of patients with GERD. It may be useful in the assessment of patients with atypical chest pain, and can be combined with an acid perfusion (Bernstein) test as well as with other provocative tests. It is recommended that manometry be performed prior to surgical intervention, because patients with significant underlying motor disorders of the esophagus (e.g., scleroderma) often develop severe dysphagia following an antireflux procedure.
7.4. Treatment
7.4.1. Medical Treatment
The treatment of GERD is directed toward the abnormal pathophysiology. The ideal therapeutic agent would be one that restores barrier function of the gastroesophageal junction. Unfortunately, at present there are no pharmacological agents that are capable of doing this well. The GABAB receptor agonist baclofen has been shown to decrease the frequency of transient LES relaxations and thereby reduce GE reflux. This drug is limited by side effects and is not yet approved for use in GERD. Prokinetic agents can increase LES pressure and improve gastric emptying and esophageal clearance, but unfortunately these drugs have fairly limited efficacy in the treatment of GERD. The one showing the most promise (cisapride) has been withdrawn from the market because of cardiac side effects.
Because of these limitations, acid suppression remains the main pharmacological approach to the treatment of GERD. It is well documented that acid and pepsin (if in an acid milieu) are the predominant constituents of refluxed gastric juice that damage the esophageal mucosa. Over the counter antacids and alginates in liquid or tablet form can alleviate heartburn symptoms when taken on an as-needed basis, and are commonly used by patients as self- medication.
Both histamine-2 receptor antagonists and proton pump inhibitors have been shown to improve symptoms and heal reflux esophagitis. The efficacy of the proton pump inhibitors is far superior to histamine-2 receptor antagonists in this regard, therefore these agents have become the mainstay of treatment for reflux disease. With a once or twice daily proton pump inhibitor treatment regimen, one can expect symptom resolution and/or healing of esophagitis in over 90% of patients.
Although the level of evidence for efficacy is not strong, certain lifestyle modifications should be considered in the management of GERD. Elevating the head of the bed on 4”-6” blocks and avoiding sleeping in the right lateral position have been shown to decrease nocturnal acid exposure. These maneuvers should be considered in patients with nocturnal reflux symptoms. Avoiding specific foods, drugs or activities may also help. Reflux is more likely to occur after large, fatty meals, especially if the patient becomes recumbent too soon after food ingestion. Certain drugs with smooth muscle-relaxing effects (e.g., calcium channel blockers, nitrates and drugs with anticholinergic effects) can decrease resting LES pressure or delay gastric emptying, and therefore may exacerbate GERD. Obesity also predisposes to GERD, therefore weight loss should be encouraged in obese patients.
GERD is a chronic relapsing condition that usually requires long-term treatment. As a general rule the physician should use the simplest, least expensive and least potent therapeutic regime that will keep the patient’s symptoms in check.
7.4.2. Antireflux Surgery
Although several different surgical procedures have been used to treat GERD, the most popular one is the “Nissen fundoplication,” originally described by the Swiss Rudolf Nissen in 1955 (Figure 8). Some expert surgeons have reported that this 360-degree gastric wrap can produce long-term control of reflux symptoms in > 90% of patients. However, more recent reports suggest that reflux symptoms eventually recur in up to 30% of patients. The Nissen fundoplication was first performed laparoscopically in 1991, and when compared to the open procedure, this approach results in reduced postoperative pain, hospital stay and recovery period, with similar functional outcome. Surgical therapy improves the LES barrier and is recommended for patients with proven gastroesophageal reflux whose symptoms respond inadequately to medical therapy, or who cannot or will not take the required medication. Ideal patients for the Nissen fundoplication are young and have an incompetent LES with normal esophageal peristaltic contraction amplitude, esophagitis documented by endoscopy and/or endoscopic biopsy and 24-hour esophageal pH monitoring demonstrating frequent reflux. Patients who should not be considered for surgical therapy include those who refuse testing, have certain primary esophageal motility disorders, have not responded initially to a trial of proton pump inhibitors, or who have normal 24-hour pH tests.
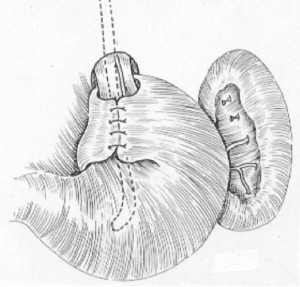
Careful diagnostic evaluation is required in all patients prior to antireflux surgery. Endsocopy determines the presence and severity of esophagitis and excludes Barrett’s esophagus, while 24-hour esophageal pH monitoring objectively documents the frequency and duration of reflux and ensures that pathological reflux is present and responsible for the patient’s symptoms. pH monitoring is a particularly important test in those patients who do not have endoscopic evidence of esophagitis. Manometry identifies the location and tone of the LES and rules out primary motility disorders of the esophagus, which might contraindicate an anti-reflux operation.
The principles of the operation are to: (1) reduce and fix the LES into the positive pressure environment of the abdomen; (2) augment the LES pressure; and (3) close the diaphragmatic hiatus around the esophagus to prevent the wrap from migrating into the chest postoperatively. Obesity, very large paraesophageal hiatal hernias, shortened esophagus and re- do antireflux surgery are relative contraindications to laparoscopic anti-reflux surgery, particularly early in a surgeon’s laparoscopic career.
7.5 Complicated GERD
7.5.1. Peptic Stricture
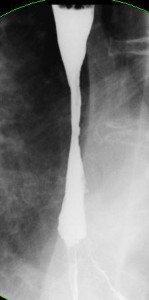
Chronic GERD may lead to peptic stricture formation (Figure 9). This is a fibrous stricture related to collagen deposition that occurs in the course of repair of esophagitis. Patients are usually asymptomatic until the luminal narrowing has reached 12–14 mm. At this point dysphagia to solids occurs. As the stricture progresses, the dysphagia gradually progresses to semisolids and then liquids. Treatment of peptic strictures involves peroral dilation, using either mercury-filled rubber bougies, rigid dilators passed over guidewires, or balloons passed through endoscopes. In close to 50% of patients one or two dilation sessions prove adequate, and no further dilations are required because ongoing medical treatment of the reflux is successful. In others, the stricture recurs and periodic dilations are required to maintain luminal patency. In patients who are otherwise healthy, consideration should be given to antireflux surgery if frequent dilations are required to maintain luminal patency. The success rate of antireflux surgery is lower in such patients with peptic stricture. Strictures are less likely to recur following dilation if the patient is treated with a proton pump inhibitor. For this reason, long-term treatment with a proton pump inhibitor is indicated in patients with peptic stricture.
7.5.2 Barrett’s Esophagus
In this condition the squamous epithelium of the distal esophagus is replaced by columnar epithelium with intestinal metaplasia (Figure 10). Deep ulcers as well as strictures at the new squamocolumnar junction may also develop. Severe hemorrhage may complicate the deep ulcers. This condition occurs in approximately 10% of patients with chronic GERD, although recent prospective studies in which careful biopsies were performed from the region of the gastroesophageal junction suggest that the incidence is may be higher.
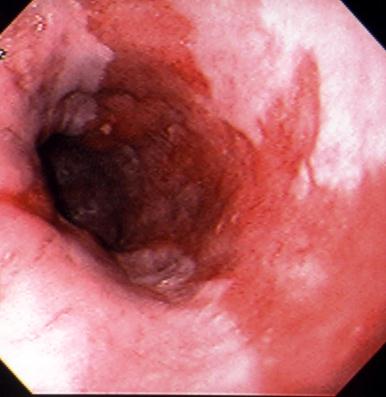
Note that broad tongues of columnar-type epithelium
extend up from the gastroesophageal junction into the
esophageal body that is normally lined with squamous
epithelium. Histology reveals an intestinal-type metaplasia
with goblet cells.
Barrett’s epithelium is a premalignant condition. At the time of initial presentation, up to 10% of patients found to have Barrett’s esophagus will have coexistent adenocarcinoma arising in the Barrett’s epithelium. This number gives an exaggerated impression of the magnitude of risk, because Barrett’s esophagus patients with cancer are more likely to seek medical attention. The true incidence of adenocarcinoma developing in Barrett’s epithelium is only about 1 case for every 200 patient-years of follow-up. This nevertheless represents about a 30- to 40-fold increase over the risk faced by the general population. For this reason current guidelines recommend that periodic (i.e., every 2-3 years) endoscopy and mucosal biopsy should be performed in order to detect precancerous lesions or early cancer. Most patients will develop severe dysplasia before frank invasive carcinoma occurs. Thus, if patients are found to have severe dysplasia or early mucosal carcinoma, esophageal resection should be considered in order to prevent the development of invasive carcinoma. Recently, photodynamic therapy, radiofrequency ablation and endoscopic mucosal resection have been introduced as effective, less invasive alternatives to surgery in patients with severe dysplasia or intramucosal carcinoma complicating Barrett’s esophagus.
7.5.3 Respiratory Complications
In some patients the refluxed gastric contents may get past the UES and into the larynx and lungs. This may produce asthma, recurrent chest infections, chronic cough and laryngitis. In addition, gastroesophageal reflux may trigger broncho-spasm or cough via a neural reflex. GERD with aspiration is more commonly seen in the pediatric age group; when present, antireflux surgery should be performed unless there is a well-documented response to medical therapy.
8. Nonreflux-Induced Esophagitis
8.1. Infectious Esophagitis
Bacteria rarely cause primary esophageal infection, although the esophagus can be involved secondarily by direct extension from the lung. The two most common forms of infectious esophagitis are caused by Candida and herpes viruses. Other viruses (e.g., CMV, HIV) and fungi can also cause esophagitis; however, this is uncommon and almost invariably associated with immunosuppression.
8.1.1. Candida Esophagitis
This is by far the most common form of infectious esophagitis. Usually there is a predisposing cause, such as diabetes mellitus, recent antibiotic therapy or some form of immunocompromise. The patient may be asymptomatic. Not all patients will have associated oral thrush. More commonly, however, patients present with odynophagia, retrosternal chest pain and/or dysphagia. Severe cases can be complicated by bleeding, a stricture and sinus tract formation with secondary lung abscess. Barium x-rays may reveal an irregular granular or even cobblestone appearance to the esophageal mucosa, but in many patients the barium esophagogram is unremarkable; for this reason, endoscopy with biopsy and brushing are required to make the diagnosis. The typical endoscopic appearance is the presence of small raised whitish plaques (Figure 11).
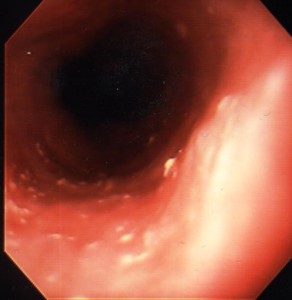
When the plaques are removed the underlying mucosa is seen to be erythematous and friable. Specimens obtained by biopsy or brush cytology should be examined microscopically for the presence of typical Candida yeast with pseudohyphae formation. Mild cases of Candida esophagitis can be treated with oral nystatin (luminal treatment); however, more extensive disease, especially if the patient is immunocompromised, may require systemic treatment with fluconazole. Amphotericin B is required if there is evidence of systemic spread.
8.1.2 Herpes Simplex Esophagitis
Next to Candida, this is the most common form of infectious esophagitis. The clinical presentation is much the same as with Candida esophagitis. There may also be constitutional symptoms of a viral upper respiratory tract infection preceding the esophageal symptoms. Herpetic mouth or skin lesions may also develop. This infection occurs most frequently in immunosuppressed patients, but also develops sporadically in healthy young adults. Endoscopy with biopsy and brush cytology is required to confirm the diagnosis. The pathognomonic finding is the eosinophilic “Cowdry’s Type A” intranuclear inclusion body. Herpetic esophagitis is self- limiting in immunocompetent individuals; specific treatment is not indicated. Symptoms of odynophagia often respond to a combination of antacids mixed with viscous Xylocaine®. In severely immunocompromised patients, intravenous acyclovir treatment should be instituted.
8.2. Eosinophilic (Allergic) Esophagitis
In recent years there has been increasing recognition of so-called allergic or eosinophilic esophagitis. It used to be felt that this was largely restricted to the paediatric population, however, adults of all ages are now being diagnosed with this disease. It is most common in young adult men. The typical presentation is recurrent solid food dysphagia and often food bolus obstructions. Barium swallow x-ray and endoscopy may show little or no change. Proximal esophageal strictures or a diffuse small caliber esophagus is a clue to this disease when seen on barium x-ray. Endoscopically (Figure 12) one often sees subtle longitudinal furrowing of the esophageal mucosa, transverse ridges or corrugation or whitish papules or plaques that have the appearance of candida esophagitis. The latter actually represent small eosinophilic abscesses. Another characteristic feature is fragility of the esophageal mucosa, such that bits of mucosa often tear away when passing the endoscope through the esophageal lumen. The diagnosis requires mucosal biopsy, which shows intense infiltration of eosinophils into the squamous mucosa. More than 15 eosinophils per high-powered field confirms the diagnosis.
Although food allergy may trigger this disorder, it is also possible that inhaled allergens may result in indirect involvement of the esophagus as part of the allergic response. It is also possible that swallowed mucus-containing inhaled allergens are responsible. A majority of these patients have a history of allergic disease such as asthma, skin atopy or allergic rhinitis. In general, allergy testing is usually unhelpful. In the paediatric population, exclusion diets and/or elemental diets have been reported to be beneficial. Currently, the preferred treatment in adults is either topical steroids (fluticasone, which is swallowed rather than inhaled) or the leukotriene inhibitor montelukast sodium.
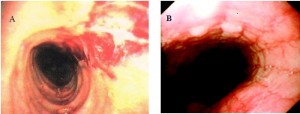
8.3. Esophagitis Associated with Immune-Mediated Disease
Rarely, esophagitis can occur in association with Crohn disease or Behçet’s syndrome. The typical lesion is scattered aphthous-type ulcerations, although severe transmural involvement with stricture formation can occur. The esophagus can also be severely involved in pemphigoid, pemphigus, epidermolysis bullosa and lichen planus. Esophagitis occurs in as many as one-third of patients who develop chronic graft-versus-host disease after bone marrow transplantation. The typical lesion is a generalized epithelial desquamation of the upper and middle esophagus. There may be associated ring-like narrowings or strictures due to submucosal fibrosis. A nonspecific esophageal motor disorder may also develop and result in superimposed reflux esophagitis because of poor esophageal clearing. Sarcoidosis also rarely causes esophageal inflammation.
8.4. Chemical-Induced Esophagitis
8.4.1. Caustic Chemical Ingestion
Strong acids or alkalis ingested accidentally or as a suicide attempt cause marked esophagitis. Alkali tends to be more injurious to the esophageal mucosa than acid and produces liquefaction necrosis as well as thermal burns (due to heat release when the alkali is hydrated by gut secretions). Acids tend to produce superficial coagulation necrosis and eschar formation. Typically the patient develops immediate chest pain and odynophagia. Oral burns also may produce local pain and drooling. There may be respiratory symptoms such as stridor, dyspnea and hoarseness if the airway is contaminated. Symptoms alone do not permit accurate prediction of the presence or absence of esophageal injury; therefore early diagnostic endoscopy should be considered in most patients. Clearly, endoscopy should not be performed if there is evidence of esophageal perforation. In the management of these patients, it is imperative to maintain an adequate airway. Oral intake must be stopped and intravenous fluids administered. Empiric treatment classically has involved antibiotics and corticosteroids, but there is no good evidence documenting the efficacy of this approach. Patients who survive the acute phase of the injury are at risk of developing strictures because of the intense collagen deposition associated with healing. This often requires repeated esophageal dilation to maintain luminal patency.
Lye-induced injury increases the risk of developing squamous cell carcinoma of the esophagus. Typically there is a 30- to 50-year lag time before the development of cancer. For this reason any patient with previous lye injury and new esophageal symptoms should be promptly investigated. The extent of the risk is such that most experts do not recommend periodic endoscopic surveillance.
8.4.2. Pill-Induced Esophagitis
A large number of oral agents can cause localized esophageal injury. The antibiotic doxycycline and the anticholinergic emepronium bromide are two of the most common culprits. Nonsteroidal anti-inflammatory drugs and slow-release forms of potassium chloride are also frequently implicated. Patients with this type of injury typically take their medication with a small amount of water and then immediately lie down to go to bed. They may then wake up several hours later with severe retrosternal chest pain and odynophagia. Capsules and tablets are notorious for being transported through the esophagus quite poorly unless adequate amounts of fluid are ingested at the same time. This is an important point to remember in counselling all patients who take medicines at bedtime. Rarely, the medication becomes lodged and causes a deep esophageal ulcer with perforation. More commonly the ulceration is superficial and heals in a few weeks. Late stricture formation may occur. Patients with esophageal motility disorders are particularly prone to this complication. The bisphosphonate alendronate sodium has also been reported to rarely cause esophageal ulceration, but the mechanism of this injury is unclear.
8.5. Radiation-Induced Esophagitis
When included in the field of irradiation, the esophagus becomes inflamed in up to 80% of patients receiving therapeutic radiation for cancer. The risk of esophagitis is greater if there is concomitant chemotherapy. The patients typically develop chest pain, dysphagia and odynophagia shortly after the initiation of therapy. This can be a serious problem in such patients, who are often already severely malnourished. Late stricture formation is a well recognized complication.
9. Disorders of the Oropharyngeal Phase of Deglutition
A variety of structural and functional disorders can disrupt the oropharyngeal phase of deglutition and result in oropharyngeal or “transfer”-type dysphagia (Table 1). In the assessment of these patients it is important to exclude disorders for which specific treatment is available. The most important investigation is a carefully performed video fluoroscopic study of the swallowing mechanism. In addition to the usual barium studies, it is helpful to observe deglutition when the patient swallows barium soaked cookies or bread. Not only will this examination identify and characterize disorders of oropharyngeal coordination, it will also help exclude structural lesions. If an inflammatory, neoplastic or other structural lesion is suspected, direct or indirect laryngoscopy is indicated. At present, manometric studies of the pharynx and UES add little to what can be learned from radiologic studies.
Ideally, treatment of oropharyngeal motor disorders should be directed at the underlying disease. Frequently this is not possible, and nonspecific treatment must be instituted. In some cases reassurance and education are all that is required. Many patients will be able to control their symptoms simply by eating slowly and carefully in a relaxed atmosphere. In patients in whom aspiration develops because of inadequate clearing of the hypopharynx after the initial swallow, it is beneficial to have the patient immediately follow a “bolus” swallow with a second,
“dry” swallow. Correcting denture problems and avoiding foods of certain consistency may also help. Speech-language pathologists have special expertise as swallowing therapists and can be very helpful in the management of these patients.
A normal tracing is on the left and depicts sequential “peristaltic” contractions in the esophageal body with full LES relaxation. Hypertensive peristalsis or “nutcracker” esophagus is characterized by normal peristalsis and LES relaxation, but the amplitude of contraction in the distal esophagus is abnormally high (> 180 mmHg). In diffuse esophageal spasm, normal peristaltic waves are interspersed with high-pressure, nonpropulsive (simultaneous) contraction waves and are often repetitive. The resting LES pressure may be abnormally high, but swallow- induced LES relaxation is normal. In achalasia there is complete absence of normal peristalsis in the smooth-muscle esophagus (simultaneous contractions only) and swallow-induced LES relaxation is either absent or incomplete. Note also that resting intraesophageal pressures are elevated. Scleroderma is characterized by the presence of weak, nonperistaltic esophageal contractions and a markedly hypotensive LES that relaxes normally with swallowing.
For patients in whom these simple measures are not helpful and whose symptoms are
such that respiratory and nutritional complications are developing, cricopharyngeal myotomy is sometimes performed. This helps patients with true cricopharyngeal achalasia or Zenker’s diverticulum (Section 13). Unfortunately, the response to myotomy is inconsistent in most other patients with oropharyngeal dysphagia, because inadequate opening of the UES is rarely due to dysfunction of the cricopharyngeal muscle alone. More often there is associated weakness of the suprahyoid muscles, which actually open the sphincter, and/or associated problems with pharyngeal peristalsis. Cricopharyngeal myotomy does little to improve such altered physiology. Once cricopharyngeal myotomy has been performed, the patient has lost an important defense mechanism against the aspiration of refluxed material. The patient should therefore be instructed to elevate the head of his or her bed on blocks in order to minimize this risk. For this same reason patients with severe GERD should not undergo cricopharyngeal myotomy unless the reflux can be controlled.
Table 1. Classification of disorder causing oropharyngeal dysphagia
Ø Central nervous system disease
o Cerebrovascular accident (brainstem, pseudobulbar palsy)
o Wilson’s disease
o Amyotrophic lateral sclerosis
o Brainstem neoplasm
o Tabes dorsalis
o Parkinson’s disease
Ø Peripheral nervous system disease
o Bulbar poliomyelitis
o Miscellaneous peripheral neuropathies
o Head and neck neoplasms
o Past-radical neck surgery
Ø Muscle disease
o Muscular dystrophy
o Polymyositis and dermatomyositis
o Metabolic myopathy (e.g., hypo- and hyperthyroidism)
o Amyloidosis
o Systemic lupus erythematosus
o Myasthenia gravis
Ø Local disorders
o Oropharyngeal inflammation
o Oropharyngeal neoplasms
o Zenker’s diverticulum
Ø Idiopathic conditions
o Cricopharyngeal achalasia
o Idiopathic oropharyngeal incoordination
When all other measures fail and nutritional and respiratory complications develop, a gastrostomy feeding tube should be placed.
10. Motor Disorders of the Esophagus and Lower Esophageal Sphincter
Esophageal motor disorders can be classified as either primary or secondary. Primary disorders refer to those that usually affect the esophagus alone and have no known etiology. Secondary disorders are motility derangements caused by some other systemic or local condition. Examples of secondary disorders include acid-reflux-induced dysmotility, dysmotility related to the neuropathy associated with diabetes and motor dysfunction secondary to esophageal involvement in scleroderma or other connective tissue disorders. The well-defined primary motor disorders include the hypertensive peristaltic or “nutcracker” esophagus, diffuse esophageal spasm and achalasia (Figure 13).
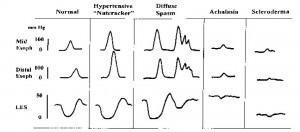
Many cases of primary motility disorders are actually “nonspecific,” having a variety of abnormalities that do not fulfill criteria established for the well-defined esophageal motor disorders. Patients with primary motor disorders typically present with dysphagia and/ or chest pain. The pain is often qualitatively similar to angina pectoris and has been classically attributed to smooth-muscle spasm. However, recent studies have suggested that the pain may be secondary to a lowered sensory threshold to esophageal stimuli such as distention or acid. Some patients with motor disorders will have secondary GERD because of poor clearing or poor LES function. Here, heartburn and regurgitation may be prominent symptoms. The diagnosis of a motor disorder can be made on the basis of history and barium swallow x-ray and endoscopy. If there is dysphagia referred to the retrosternal area and no evidence of a structural lesion or inflammatory disease on x-ray or endoscopy, then by exclusion the patient’s dysphagia is likely related to a motor disorder. As mentioned previously, the quality of the dysphagia (e.g., sporadic, unpredictable dysphagia to both liquids and solids) is also helpful in differentiating motor disorders from structural causes of dysphagia. During fluoroscopy, the radiologist is usually able to detect abnormalities of motor function as the barium is swallowed. The use of a solid bolus, such as a piece of bread soaked in barium, may be helpful in diagnosing esophageal rings or webs. Endoscopy primarily rules out secondary causes of the disorder (i.e., reflux or eosinophilic esophagitis and neoplasm). In order to define specifically the type of motor disorder present, however, esophageal motility studies are required. The manometric features of the important esophageal motor disorders are depicted schematically in Figure 9.
10.1. “Nutcracker” Esophagus
This motility disorder is characterized by normally propagated but high amplitude peristaltic waves in the distal esophagus. The duration of the contraction wave is also often prolonged. LES relaxation is normal, although in many patients the resting LES pressure is elevated. Patients often present with angina-like chest pain and usually do not complain of dysphagia. Nutcracker esophagus is the most frequent abnormal manometric finding in patients referred for evaluation of noncardiac angina-like chest pain. The etiology is unknown. Rarely, this disorder progresses to diffuse esophageal spasm or even vigorous achalasia. Reassurance that the pain is not cardiac but is secondary to a benign esophageal condition is the most important part of treatment. Nitrates and calcium channel blockers (to relax smooth muscle) have been used extensively, but have no proven benefit. Tricyclic antidepressant drugs are effective in alleviating the pain in these patients, presumably because of their effect on visceral sensation. In some patients with nutcracker esophagus, pain is actually triggered by acid reflux; these patients often respond dramatically to appropriate antireflux therapy.
10.2. Diffuse Esophageal Spasm
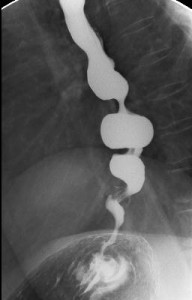
Figure 14. Barium contrast X-ray depicting a “Corkscrew” esophagus, typical of diffuse esophageal spasm. Simultaneous contractions at multiple sites along the esophagus create this pattern. A similar X-ray picture may be seen in vigorous achalasia, therefore manometry is required to firmly establish the diagnosis.
This is characterized by normal peristalsis interspersed with frequent high pressure nonpropagated or “tertiary” waves and multipeaked waves. Patients often present with dysphagia and chest pain. In advanced diffuse esophageal spasm, the x-ray will show a corkscrew pattern (Figure14) as different segments of the esophagus vigorously and simultaneously contract. The etiology is obscure, but may relate to degenerative changes in the intrinsic and extrinsic esophageal nerves. Management involves reassurance and the use of nitrates or calcium channel blocking agents. Injection of Botulinum toxin into the muscle of the LES or distal esophagus also appears to be effective in alleviating dysphagia in this condition. Rarely, patients with severe disease unresponsive to medical measures may benefit from a long esophageal myotomy.
10.3. Achalasia
This uncommon primary motility disorder is characterized by aperistalsis in the body of the esophagus and absent or incomplete LES relaxation in response to swallowing. Resting LES pressures may also be elevated. Failure of LES relaxation leads to progressive proximal dilation of the esophagus with consequent elevated resting intraesophageal pressures. On x-ray the esophagus is dilated, and retained food and fluid may be present. The distal esophagus narrows in a beak-like fashion (Figure 15). This “beak” represents the hypertonic, nonrelaxing LES. In some patients there are associated high amplitude nonperistaltic contractions in the esophageal body, a condition called vigorous achalasia. Achalasia is caused by an inflammatory reaction directed against the inhibitory nitric oxide neurons within the esophageal and LES myenteric plexus. Nerve damage may also be found in the vagal nerve trunks and the dorsal motor nuclei, although these are likely secondary to the myenteric plexus damage. The parasite Trypanosoma cruzi, which is endemic in Brazil, can cause achalasia by destroying myenteric neurons (Chagas’ disease). Neoplastic disease can also interfere with esophageal and LES nerve function and cause secondary or “pseudo” achalasia. In most cases, however, the cause of the degeneration is unknown. The cardinal symptom of achalasia is dysphagia, although chest pain and even heartburn may be present. The heartburn is usually not due to gastroesophageal reflux. It may be caused by lactic acid formed by fermentation of stagnant esophageal contents. Another common symptom of achalasia is regurgitation of esophageal contents. Patients with achalasia secondary to cancer are typically older and present with rapidly progressive dysphagia and significant weight loss.
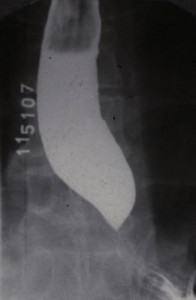
In mild cases of idiopathic achalasia treatment can begin with the use of calcium channel blockers or long-acting nitrates, which have been shown to decrease LES pressure. This is rarely successful in the long term, however. The treatment then usually performed is pneumatic balloon dilation of the LES. This consists of passing a balloon across the sphincter and inflating it rapidly so that the sphincter is forcefully dilated. Pneumatic dilation is successful in alleviating the dysphagia and improving esophageal transport in 60–90% of patients, although repeated dilations are often required to achieve the highest success rate. Patients who do not respond to pneumatic dilation should be treated with Heller myotomy. This consists of a longitudinal incision through the muscle of the LES, which is now done via a laparoscopic approach. Increasingly, laparoscopic Heller myotomy is being offered as first-line therapy in patients with achalasia. Following either pneumatic dilation or Heller myotomy, the patient can develop GERD, because the pressure barrier preventing reflux has been destroyed. This tends to be worse after Heller myotomy and has led some surgeons to perform a modified antireflux procedure at the time of myotomy. Recent studies have found that injection of botulinum toxin into the muscle of the LES can alleviate dysphagia in approximately two-thirds of patients with achalasia. This therapy is limited because the response is not sustained (average duration is approximately one year), but it may be a useful treatment option in elderly patients who would not tolerate the complications of more invasive therapy. Achalasia patients have an increased risk of developing esophageal cancer and need to be carefully evaluated if new esophageal symptoms develop. Unlike Barrett’s esophagus, regular endoscopic surveillance is not recommended in achalasia patients.
10.4. Scleroderma Esophagus
Patients with scleroderma frequently have esophageal involvement. This may occur even in the absence of obvious skin and joint involvement, although in such cases, Raynaud’s phenomenon is almost always present. The initial event is damage to small blood vessels, which in turn leads to intramural neuronal dysfunction. With time, actual muscle damage and fibrosis occur. This results in a very hypotensive LES, as well as weak, nonpropulsive esophageal contractions. Scleroderma may also involve the stomach and cause delayed gastric emptying. As a result, patients develop gross GERD. They present with heartburn and regurgitation, as well as dysphagia. The dysphagia can be due to poor esophageal propulsion and/or reflux-induced stricture. These patients need very aggressive treatment for GERD, often requiring twice-daily PPI therapy. Because they have very poor peristaltic function, increasing the barrier at the LES with antireflux surgery may markedly worsen the dysphagia.
11. The Esophagus as a Cause of Angina-Like Chest Pain
At least one-third of the patients referred to a cardiologist or admitted to a coronary care unit because of angina-like chest pain will have cardiac causes excluded. Because in most of these patients an alternative etiology is not apparent, they are often labeled as having “noncardiac chest pain.” Lack of a specific diagnosis may lead to ongoing anxiety, changes in lifestyle and frequent medical consultations if the patient continues to worry that serious heart disease may be present. In such patients esophageal disease or dysfunction should be considered. The pathophysiology of angina-like chest pain of esophageal origin is poorly understood. In some patients acid reflux is the cause: these patients experience angina-like chest pain under circumstances in which most people would experience heartburn. In others, the pain is caused by abnormal “spastic” contractions of the esophagus that either occur spontaneously or are secondary to acid reflux. These contractions may be confined to the longitudinal smooth muscle layer, therefore would not be detectable using conventional intraluminal manometry. Many of these patients appear to have an abnormal esophageal pain threshold; pain episodes may be triggered by multiple different stimuli that in normal subjects would not be perceived as painful. The diagnostic approach to patients with noncardiac chest pain is controversial. In the past, full esophageal testing was usually recommended, including upper GI endoscopy, esophageal manometry with provocative testing (Figure 16) and/or 24-hour ambulatory esophageal pH monitoring (Figure 4). More recently the value of such testing has been called into question.
Endoscopy is performed primarily to look for evidence of reflux esophagitis, but the diagnostic yield in this setting is low, and a negative result does not rule out acid reflux as a cause of pain. Esophageal manometry with “provocative testing” (e.g., esophageal acid perfusion, balloon distention or administration of muscarinic agonist) may be used in an attempt to reproduce the patient’s chest pain and possibly relate it to induced esophageal muscle spasm.
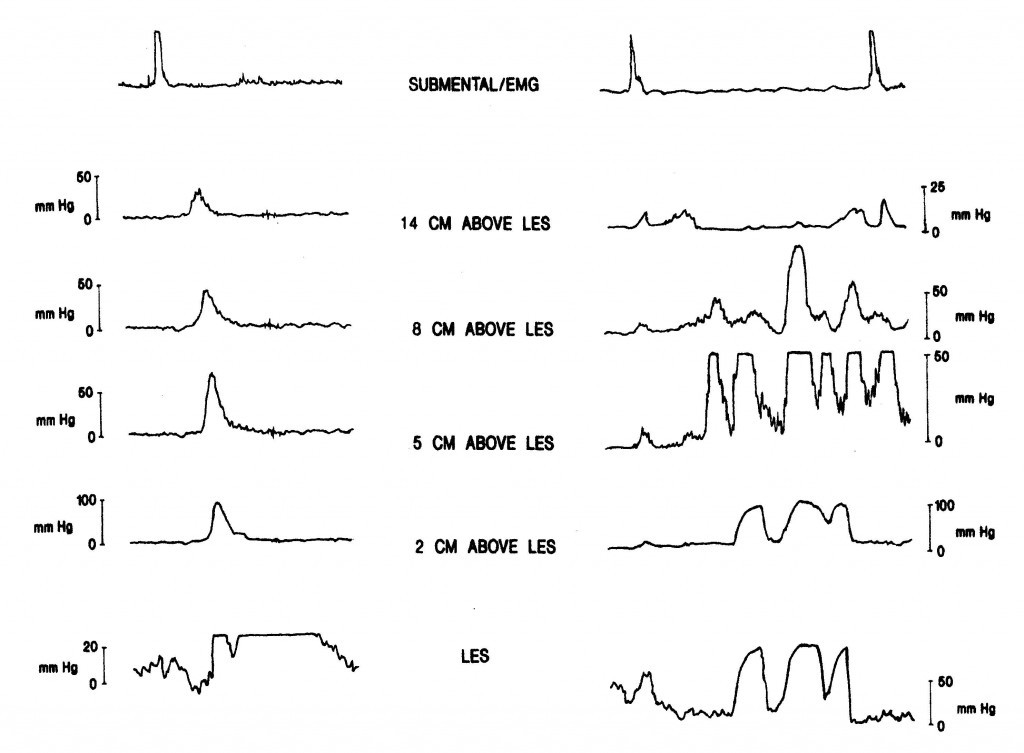
Source: Paterson WG, Marciano-D’Amore DA, Beck IT, et al. Esophageal manometry with provocative testing in patients with non-cardiac angina-like chest pain. Canadian Journal of Gastroenterology1991; 5(2):51–57. Reproduced with permission of the Canadian Journal of Gastroenterology.
However, this test appears to lack specificity, as the patient with a positive provocative test may experience seemingly identical spontaneous pain episodes that are unrelated to esophageal dysfunction. Ambulatory 24-hour pH monitoring can be extremely useful in correlating pain episodes with reflux events, but patients must have frequent (i.e., daily) pain attacks if one is likely to be captured during the monitoring period. Because GERD is probably the most common, specifically treatable cause of noncardiac chest pain, it has been recommended that these patients first receive intensive treatment for GERD (i.e., twice-daily proton pump inhibitor therapy). If symptom resolution occurs, then a diagnosis of reflux-induced pain can be presumed and the patient managed accordingly. More in-depth esophageal testing can then be reserved for those patients who fail this empiric therapy and have persisting troublesome pain, especially if associated with considerable anxiety surrounding the diagnosis. Management of angina-like chest pain of esophageal origin should be directed at the specific pathophysiological process. If the pain is triggered by gastroesophageal reflux, then antireflux treatment may be quite helpful. If the pain is due to esophageal spasm, smooth-muscle relaxants such as nitrates and calcium channel blockers may help, although few controlled clinical trials have demonstrated any significant benefit. Tricyclic antidepressants in relatively low dosage have been shown to be beneficial and should be tried in patients with frequent pain episodes that are not caused by reflux or severe esophageal spasm. These are most likely to be useful in patients with abnormal visceral nociception, or the so-called irritable esophagus. Simple reassurance and education are probably the most important part of treatment. Symptoms often improve once the patient is given a positive diagnosis and no longer fears that underlying heart disease is the cause.
12. Esophageal Neoplasms
A large number of different tumors can involve the esophagus (Table 2). The vast majority are extremely rare and often do not produce clinical disease.
Table 2. Classification of esophageal tumors
Ø Benign tumours
o Epithelial origin
− Squamous cell papilloma
o Non-epithelial origin
− Leiomyoma
− Granular cell tumor
− Hemangioma
− Lymphangioma
Ø Malignant tumors
o Epithelial origin
− Squamous cell carcinoma
− Adenocarcinoma
− Adenoid cystic carcinoma
− Mucoepidermoid carcinoma
− Adenosquamous carcinoma
− Undifferentiated carcinoma; small-cell carcinoma
o Non-epithelial origin
− Leiomyosarcoma
− Carcinosarcoma
− Malignant melanoma
o Secondary tumors
− Malignant melanoma
− Breast carcinoma
Ø Tumor-like lesion
o Fibrovascular polyp
o Heterotopia
o Congenital cyst
o Glycogen acanthosis
Carcinoma of the esophagus is a relatively uncommon malignancy in Canada, with only 3 to 4 new cases per 100,000 population per year in males and just over 1 new case per 100,000 population per year in females. Nevertheless, because of its poor prognosis, esophageal cancer ranks among the 10 leading causes of cancer death in Canadian men 45 years of age and older. Although several different types of primary and secondary malignancies can involve the esophagus (Table 2), squamous cell carcinoma and adenocarcinoma are by far the most common esophageal malignancies.
12.1. Adenocarcinoma
Adenocarcinoma used to make up approximately 10% of all esophageal cancers. However, its incidence has been increasing in recent decades such that now it comprises up to 40–60% of esophageal cancers in North America. Rarely, primary esophageal adenocarcinomas arise from embryonic remnants of columnar epithelium or from superficial or deep glandular epithelium. In most instances, adenocarcinoma arises from metaplastic Barrett’s epithelium in the distal esophagus (Figure 17). Adenocarcinoma of the cardia of the stomach may also involve the distal esophagus and give the appearance that the cancer arises from the esophagus.
The true incidence of Barrett’s-related cancer is uncertain, but most studies suggest that patients with Barrett’s esophagus will develop adenocarcinoma at a rate of about 0.5% per year. This is a significant problem given the large number of reflux patients with Barrett’s metaplasia. Because dysplasia develops prior to frank carcinoma in Barrett’s epithelium, current guidelines recommend that these patients should undergo surveillance endoscopy with multiple biopsies every 2-3 years to identify those who are likely to progress to cancer (Section 7).
The clinical presentation and diagnostic evaluation of patients with adenocarcinoma of the esophagus are similar to those of squamous cell carcinoma (Section 12.2.2). Neoadjuvant therapy with concomitant radiation and chemotherapy followed by surgical resection of the esophagus has a 13% absolute benefit in survival at 2 years versus surgery alone. Surgical resection may be performed with curative intent. However, surgical resection for palliation, or palliation with laser, photodynamic therapy, peroral dilation and/or stent placement are more often required, since curative surgery is feasible in only 20% of patients. The prognosis is similar to that for gastric adenocarcinoma – i.e., an overall five-year survival rate of < 10%.
Table 3. Esophageal squamous cell carcinoma: possible factors
o Alcohol
o Tobacco
o Nutritional exposure
− Nitrosamines: “bush teas” containing tannin and/or diterpene phorbol esters
o Nutritional deficiencies (riboflavin, niacin, iron)
o Chronic esophagitis
o Achalasia
o Previous lyle-induced injury
o Tylosis
o Plummer Vinson (Paterson-Kelly) syndrome
12.2. Squamous Cell Carcinoma
The occurrence of squamous cell carcinoma of the esophagus shows striking geographic variability, with high frequencies in certain regions of Iran, Africa, China and the former USSR. This has led to several theories concerning certain environmental agents that may be important etiologically (Table 3). In North America, squamous cell carcinoma is associated with alcohol ingestion, tobacco use and lower socioeconomic status. It is also significantly more common in blacks and in males.
Characteristically these cancers, similarly to adenocarcinoma, extend microscopically in the submucosa for substantial distances above and below the area of the gross involvement. They also have a propensity to extend through the esophageal wall and to regional lymphatics quite early. Furthermore, they usually produce symptoms only when they have become locally quite advanced. For these reasons approximately 95% of these cancers are diagnosed at a time when surgical cure is impossible.
In most studies, the mid-esophagus is the most common site of origin; however, others have reported distal cancers to be most common. The lungs, liver and bones are the most common sites of distant metastases.
Most patients present with progressive, predictable dysphagia and weight loss. Other symptoms include odynophagia, chest pain (which may radiate to the mid-scapular region), hoarseness (due to recurrent laryngeal nerve involvement) and blood loss. Pulmonary complications due to either direct aspiration or esophagorespiratory fistulas are also quite common during the course of the disease. Physical examination is usually negative aside from signs of weight loss. Hepatomegaly or enlarged cervical or supraclavicular lymph nodes may be detected in cases of disseminated metastases.
Barium swallow is usually diagnostic, although small cancers can be missed in up to 30% of cases. Endoscopy with multiple directed biopsies combined with brush cytology is required to confirm the diagnosis. This should be followed by careful attempts to stage the disease prior to deciding on therapeutic intervention. CT scan of the thorax and upper abdomen is required to look for local spread and metastasis. Unfortunately the CT scan lacks sensitivity in this regard. Endoscopic ultrasound appears promising in accurately assessing depth of tumor involvement and presence or absence of enlarged mediastinal lymph nodes. PET scanning is useful to delineate lymph nodes positive for metastatic malignancy.
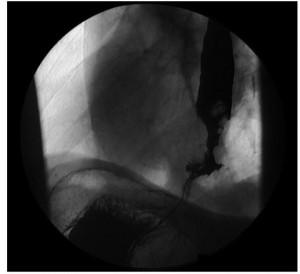
Treatment results of squamous cell carcinoma of the esophagus are discouraging. These tumors are quite radiosensitive; however, most centers give radiotherapy to patients who have advanced unresectable tumors or other health problems that make them poor surgical candidates. This understandably leads to very poor overall survival following radiotherapy. In the few reports where radiotherapy is used as the primary mode of therapy in patients who might otherwise be considered surgical candidates, the five-year survival rate is as high as 17%, which compares quite favorably to surgical results. Both forms of treatment have significant morbidity, but the surgical mortality following esophageal resection is 5–10%. Controlled trials are needed, but in only a small proportion of the total population of esophageal cancer patients is cure a realistic goal. In the majority the disease is too far advanced. New regimens that combine radiotherapy and chemotherapy, with or without surgery, are currently being evaluated and show promise in improving cure rates and disease-free survival. However, the toxicity and morbidity from combined treatment can be substantial.
The goal of treatment has to be palliation in most patients. Both radiotherapy and palliative surgery can be used in this setting; however, other modalities are often necessary. The dysphagia can be relieved with peroral dilation, but in many patients this becomes exceedingly difficult as the disease progresses. If this is the case, a prosthetic device can sometimes be placed across the tumor to maintain luminal patency. These stents can work quite well, although tube blockage, tube migration, erosion through the esophageal wall and sudden massive aspiration are important complications. These prosthetic devices are the best treatment for an esophagorespiratory fistula. Photodynamic therapy and radiofrequency ablation are two relatively new minimally invasive treatment modalities for palliating esophageal cancer. The former involves using a photosensitizing compound that accumulates in cancer cells, which leads to their destruction when they are exposed to light of a certain wavelength. The caring physician must also provide emotional support, nutritional support and adequate pain therapy for these unfortunate patients.
13. Miscellaneous Disorders of the Esophagus
13.1. Webs and Rings
Webs are thin, membrane-like structures that project into the esophageal lumen. They are covered on both sides with squamous epithelium and are most commonly found in the cervical esophagus. Webs are usually detected incidentally during barium x-rays and rarely occlude enough of the esophageal lumen to cause dysphagia. The etiology of these webs is unclear. Most are probably congenital in origin. In some instances postcricoid esophageal webs are associated with iron deficiency and dysphagia – the so-called Plummer-Vinson or Paterson-Kelly syndrome. This syndrome is associated with increased risk of hypopharyngeal cancer and should be managed with bougienage, iron replacement and careful follow-up. Esophageal webs may also form after esophageal injury, such as that induced by pills or lye ingestion, and have also been reported in association with graft-versus-host disease. The lower esophageal or Schatzki’s ring is also a membrane-like structure, but unlike webs is lined by squamous epithelium on its superior aspect and columnar epithelium inferiorly. Such a ring is quite common, being detected in up to 10% of all upper GI barium x-rays. Few produce sufficient luminal obstruction to cause dysphagia (yet a lower esophageal ring is a common cause of dysphagia). When the lumen is narrowed to a diameter of 13 mm or less, the patient will experience intermittent solid-food dysphagia or even episodic food-bolus obstruction. Treatment of a symptomatic Schatzki’s ring involves shattering the ring with a large-diameter bougie or a balloon dilator. Subsequent treatment with a proton pump inhibitor has been shown to decrease the recurrence of symptomatic Schatzki’s rings.
13.2. Diverticula
Pharyngoesophageal diverticula are outpouchings of one or more layers of the pharyngeal or esophageal wall and are classified according to their location.
13.2.1. Zenker’s Diverticulum (Figure 18)
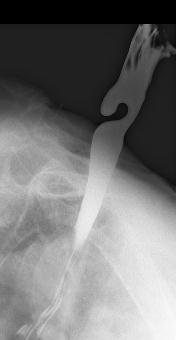
This diverticulum arises posteriorly in the midline between the oblique and transverse (cricopharyngeal) fibers of the inferior pharyngeal constrictor muscles. As this diverticulum enlarges, it usually shifts to the left of the midline. Zenker’s diverticulum forms because of decreased compliance of the cricopharyngeal muscle, which results in abnormally high pressures in the hypopharynx during deglutition. In addition to pharyngeal-type dysphagia, Zenker’s diverticulum may be associated with effortless regurgitation of stagnant, foul-tasting food, as well as aspiration. A very large diverticulum can produce a neck mass, usually on the left side.
Treatment of a symptomatic Zenker’s diverticulum is surgical. Most surgeons will either resect the diverticulum or suspend it (diverticulopexy) so that it cannot fill. This is combined with cricopharyngeal myotomy. In many cases, particularly if the diverticulum is small, cricopharyngeal myotomy alone will alleviate symptoms. This procedure can now be performed endoscopically in selected cases. Once the cricopharyngeal myotomy has been performed, the patient has lost an important defense mechanism to prevent the aspiration of refluxed material.
The patient should therefore be instructed to elevate the head of the bed in order to minimize this risk. For the same reason patients with severe GERD should not undergo cricopharyngeal myotomy unless the reflux can be controlled either medically or surgically.
13.2.2. Midesophageal Diverticula
Traditionally, midesophageal diverticula have been called “traction” diverticula because of their supposed etiology. They were believed to arise secondary to old mediastinal inflammation, such as tuberculosis, that caused adherence of mediastinal structures to the outer esophageal wall so that outward traction occurred during peristalsis. It now appears likely that very few midesophageal diverticula arise this way. In most there is an associated motility disorder and it is likely that this is actually a “pulsion” diverticulum formed when a peristaltic wave deteriorates into a simultaneous or spastic contraction in the smooth-muscle esophagus. Midesophageal diverticula rarely require specific therapy. Rather, the associated motor disorder requires treatment if symptomatic.
13.2.3. Lower Esophageal or Epiphrenic Diverticula
These “pulsion” diverticula form just above the LES and are invariably associated with an esophageal motor disorder – usually diffuse esophageal spasm, with or without abnormal relaxation of the LES. Patients with these diverticula usually present with dysphagia and/or angina-like chest pain. In addition, they may complain of nocturnal regurgitation of large quantities of stagnant fluid. If symptoms are present, treatment with nitrates or calcium channel blockers may be helpful. If this is not successful, surgery is indicated. Any surgical attack on these diverticula should involve a myotomy of the spastic distal esophagus and/or LES. Resection of the diverticula alone seldom affords long-term benefit.
13.2.4. Intramural Diverticulosis
This disorder has a characteristic radiologic appearance consisting of numerous tiny, flask-shaped outpouchings from the esophageal lumen. There is usually an associated smooth stricture in the proximal esophagus. Patients typically present with dysphagia that responds to peroral dilation. The outpouchings are actually dilated ducts coming from submucosal glands and thus are not true diverticula. The etiology is obscure. Some cases are associated with esophageal candidiasis, but this organism does not appear to be of etiological importance.
13.2.5. Esophageal Trauma
Blunt or penetrating trauma to the chest can cause esophageal injury. In addition, esophageal instrumentation such as that used in bougienage, endoscopy or stent insertion may cause perforation or mucosal laceration. Severe retching or vomiting can also cause esophageal perforation (B oerhaave’s syndrome) or mucosal laceration (Mallory-Weiss tear). Boerhaave’s syndrome is a life-threatening condition that requires immediate surgery to drain the mediastinum and repair the defect in the esophageal wall. Patients, typically alcoholics, present with sudden epigastric and/or chest pain following a bout of vomiting and usually have fever and signs of hypovolemia or shock. The diagnosis is established by having the patient swallow a small amount of water-soluble contrast material (e.g., Gastrografin®), which is seen to leak into the mediastinum or pleural cavity through the esophageal perforation. CT of the chest may also reveal an esophageal perforation. If the perforation is large, a chest x-ray may be diagnostic.
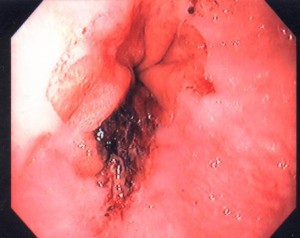
The mucosal laceration of the ‘Mallory- Weiss’ tear (Figure 19) is probably better classified as a disorder of the stomach, because in most cases the laceration starts at the GE junction and extends down into the stomach. These patients present with hematemesis or melena following a bout of retching or vomiting. The bleeding usually stops spontaneously and only supportive therapy is required. If bleeding persists, endoscopically applied hemostasis or surgical intervention may be necessary.
Figure 19. Endoscopic view of a Mallory-Weiss tear. Note the mucosal laceration with blood clot at its base at the gastroesophageal junction. Patients with this lesion typical have vigorous retching or vomiting before vomiting up fresh blood and/or passing melena.
13.3. Food-Bolus Obstruction and Foreign Bodies
A surprising variety of foreign bodies can lodge in the esophagus after being swallowed either inadvertently or deliberately. The three most common sites where foreign bodies become stuck are the piriform sinuses, at the aortic arch and just above the LES. The patient can usually localize the site of the obstruction quite accurately, and this can be confirmed using routine x- rays if the object is radiopaque. Most foreign bodies can be removed by an expert endoscopist. Surgery is rarely required, except when perforation has occurred. A more common clinical problem is esophageal food-bolus obstruction. This typically occurs when a patient with a motility disorder, esophagitis, stricture or Schatzki’s (lower esophageal) ring swallows a large solid-food bolus. The patient notices immediate pain, usually well localized to the site of obstruction in the chest, but sometimes referred to the suprasternal notch. Attempts to swallow anything further are unsuccessful and usually lead to prompt regurgitation. Many physicians will initially treat these patients with smooth-muscle relaxants such as intravenous glucagon or sublingual nitroglycerin; however, there is little evidence that this approach is efficacious. Drinking carbonated beverages may also help the bolus pass, presumably by distending the esophageal lumen with gas. If the food bolus does not pass on spontaneously within a few hours, endoscopy should be performed, at which time the bolus can either be removed per os or pushed through into the stomach. A persistent food bolus impaction, if left untreated for a long period (> 12-24 hours), may lead to mucosal ulceration and even a localized perforation.
Authors: W.G. Paterson, S. Mayrand and C.D. Mercer
Source: First Principles of Gastroenterology and Hepatology
 Oval Useful news from Azerbaijan and Caucasus
Oval Useful news from Azerbaijan and Caucasus


