- Introduction / N.Saloojee, W.G. Thompson and C. Dubé
- Globus / C. Dubé, W.G. Thompson and N. Saloojee
- Heartburn and Regurgitation / C. Dubé, W.G. Thompson and N. Saloojee
- Dysphagia / A.S.C. Sekar and N. Saloojee
- Odynophagia / N. Saloojee
- Dyspepsia / C. Dubé and N. Saloojee
- Nausea and Vomiting / C. Champion and N. Saloojee
- Anorexia / M.C. Champion and N. Saloojee
- Gas and Bloating / W.G. Thompson and N. Saloojee
- Constipation / C. Dubé, W.G. Thompson and N. Saloojee
- Diarrhea / W.G. Thompson and N. Saloojee
- Malnutrition / G. Patel
- Acute Abdominal Pain / J.M. Watters and N. Saloojee
- Chronic Abdominal Pain / W.G. Thompson and N. Saloojee
- Abdominal Mass / S. Grégoire and N. Saloojee
- Proctalgia Fugax / W.G. Thompson and N. Saloojee
- Oral-Cutaneous Manifestations of GI Disease / N. Saloojee
- Extraintestinal changes seen in patients with GI disease
1. Introduction / N.Saloojee, W.G. Thompson and C. Dubé
Gastrointestinal (GI) symptoms and disease are extremely common in the general population. GI symptoms and disease will be encountered by all physicians, regardless of specialty. It is therefore important for all physicians to have a framework to approach common GI complaints.
Especially in persons with GI symptoms, taking an accurate history is the key to diagnosis and effective management. In taking the history, the first priority is not to miss serious disease, for example GI malignancy. So called “alarm symptoms” need to be sought out. Dysphagia, GI bleeding, significant weight loss, fever and pain waking the patient from sleep are examples of alarm symptoms that should prompt rapid investigation to exclude a serious GI disorder.
It is important to recognize that GI symptoms may indicate organic disease or functional disease. Organic disease refers to a well defined disorder (eg peptic ulcer disease, inflammatory bowel disease, or malignancy). Patients with functional GI disorders are more common. They have symptoms, but no objective abnormality on physical examination or diagnostic testing. The most common such disorders are irritable bowel syndrome (IBS) and functional dyspepsia (FD). The carefully taken history is often helpful in distinguishing organic from functional disease.
A good interview will include an accurate description of the symptom. This will include time of onset, location, duration, character, and radiation. Aggravating or relieving factors (eg eating or defecation), review of other GI symptoms, past history of GI disease or other illness, prior surgery and family history of GI disease should be sought.
A psychosocial history is important for a number of reasons. Functional disease is more frequent in patients with a history of traumatic life events. In patients with organic disease, anxiety or psychologic stress may worsen symptomatology (eg inflammatory bowel disease). This history is also important in addressing the patient’s concerns. A physician cannot adequately address a patient’s concerns if he or she does not know what they are.
The following is a synopsis of common GI symptoms. These notes include a description of the symptom, pathophysiologic considerations, important historical features and physical exam findings, and a brief approach to diagnosis and management. Greater detail can be found in later discussions of specific diseases. The final section of this chapter presents an approach to the examination of the abdomen.
2. Globus / C. Dubé, W.G. Thompson and N. Saloojee
Globus refers to the sensation of a lump or foreign body in the throat. The sensation may be intermittent or persistent. In a study of healthy volunteers, 45 % of people have experienced this at least once in their lives (1). Globus often occurs during periods of psychologic or emotional stress. Globus is present between meals, and is not food-related. Patients with this symptom swallow normally and without pain. In other words, globus is not associated with dysphagia or odynophagia.
There is no clear etiology for globus. In the majority of cases, no underlying pathology can be identified. In some cases there is an association between globus and the presence of stress, psychiatric disorders, upper esophageal sphincter dysfunction, esophageal dysmotility, or gastroesophageal reflux disease.
Evaluation of this complaint starts with the history. Ensure that the patient does not have dysphagia, odynophagia or other alarm symptoms such as weight loss. Physical examination is generally unrevealing.
Patients with persistent globus should have an ear, nose and throat (ENT) referral. Laryngoscopy is often done to rule out significant ENT pathology. Barium swallow or endoscopy may also be done to exclude significant pathology.
If investigation is negative, globus is best managed with simple reassurance. If globus is severe, a trial of proton pump inhibitor to suppress gastric acid may be given. This is aimed at reducing acid reflux that might contribute to the symptom (2). Endoscopy is often normal in patients with acid reflux. If appropriate, psychiatric consultation may be undertaken.
3. Heartburn and Regurgitation / C. Dubé, W.G. Thompson and N. Saloojee
3.1. Description
Heartburn refers to a burning sensation experienced behind the sternum. It is due to reflux of gastric acid into the esophagus. Regurgitation is the effortless return of gastric or esophageal contents into the pharynx without nausea, retching or abdominal contractions. Heartburn and regurgitation often occur concomitantly. They are symptoms of gastroesophageal reflux disease (GERD). Heartburn and regurgitation are common symptoms, with 10-20 % of the general population experiencing this at least once weekly (3). Only a minority of people with these symptoms will seek medical advice.
3.2 History
A number of points are important in taking a history. The sensation of heartburn may radiate to the neck. It is often felt after meals, (especially fatty food, spicy food, caffeine or alcohol). It may be aggravated by lying down, bending over, or straining. Unlike angina, heartburn is not worsened by exercise or physical activity. Ischemic heart disease is often misdiagnosed as GERD, and the physician must take an especially careful history to distinguish between the two common conditions. Misdiagnosing ischemic heart disease for heartburn may have serious consequences for the patient, and medicological issues for the physician.
It is important to note that patients may mean a variety of things when they use the term heartburn. Physicians should closely question patients as to what symptom they have when they say they have heartburn, indigestion, or dyspepsia. Symptoms associated with GERD may include chest pain, epigastric pain, nausea, globus, or less commonly odynophagia. Waterbrash may be associated. Waterbrash is spontaneous hypersalivation, thought to be due to a vagal reflex triggered by excess esophageal acid. Atypical symptoms of reflux can include chronic cough, wheeze or hoarseness.
Dysphagia may be a result from esophageal dysmotility induced by esophageal acid exposure, or it may result from a reflux induced esophageal stricture. Ongoing gastroesophageal reflux is a risk factor for esophageal cancer which can result in dysphagia, weight loss, bleeding or anemia.
The approach to investigation and management of GERD will be reviewed in the chapter “Esophagus.”
4. Dysphagia / A.S.C. Sekar and N. Saloojee
4.1 Description
Dysphagia means difficulty swallowing. Dysphagia is an alarm symptom requiring prompt evaluation to exclude esophageal malignancy.
It can be classified as oropharyngeal dysphagia or esophageal dysphagia. A good history will distinguish between these two entities.
4.2. Approach to dysphagia
Oropharyngeal dysphagia is also known as “transfer” dysphagia. Patients have difficulty initiating a swallow. They may aspirate such that they cough or choke when they eat. They may have nasal regurgitation of food. Often, oropharyngeal dysphagia occurs in a patient with central nervous system pathology (eg. stroke or amyotrophic lateral sclerosis) or neuromuscular disease (eg myasthenia gravis or dermatomyositis).
Esophageal dysphagia may be due to a mechanical reason causing partial obstruction of the esophagus, or to dysmotility of the esophagus (Table 1). Less commonly, dysphagia results from extrinsic esophageal compression. Patients with esophageal dysphagia describe a sense of food or liquid sticking in the retrosternal area.
| Table 1. Causes of Esophageal Dysphagia | ||
| Mechanical Lesions | Extrinsic Lesions | Motility Disorders |
|
|
|
Certain historical points are important in evaluating esophageal dysphagia. Duration and rate of progression should be established. It is important to know if the dysphagia is to solids, liquids or both. A history of heartburn or regurgitation may point to a reflux stricture or reflux- induced esophageal dysmotility. Weight loss, bleeding or anemia could indicate esophageal malignancy. Intermittent dysphagia tends to be seen more in disorders of esophageal motility. A lower esophageal ring (Schatzki’s ring) may create intermittent dysphagia. This is because the ring is flimsy, and only sometimes delays passage of food.
Food bolus obstruction may occur in a patient with dysphagia. In this situation, a bolus of food becomes impacted in the esophagus. Patients experience chest discomfort. When they swallow liquid, it is almost immediately regurgitated. The patient will often present to the emergency department and require endoscopic removal of the food bolus. This condition is often seen in persons with eosinophilic esophagitis.
A Zenker’s diverticulum is an outpouching immediately above the upper esophageal sphincter. In addition to dysphagia, patients may experience halitosis and aspiration of food retained in the diverticulum.
If a patient is suspected to have oropharyngeal dysphagia, a videofluoroscopy swallowing study (VFSS) can confirm the diagnosis. This is a test in which swallowed contrast material is radiologically visualized. Management of oropharyngeal dysphagia involves treatment of the underlying disorder if possible and dietary modification together with the helpful guidance of a speech language pathologist.
If a patient is suspected to have esophageal dysphagia, evaluation proceeds with either an endoscopy or barium swallow. Barium swallow has the advantage of being noninvasive, however biopsies cannot be taken. If a stricture is identified at endoscopy (EGD, esophagogastroduodenoscopy), multiple biopsies are necessary to establish whether it is benign or malignant.
When a barium swallow and endoscopy fail to identify any pathology, esophageal manometry may be performed to demonstrate an esophageal motility disorder.
Treatment of esophageal dysphagia depends on the underlying cause. Benign strictures due to reflux are managed with endoscopic dilation and acid suppression in the form of a proton pump inhibitor (PPI). Benign anastomotic strictures, radiation strictures and rings are similarly treated with periodic dilation. Esophageal malignancy is managed through a combination of surgery, radiation, chemotherapy and sometimes with the insertion of a palliative endoscopic stent. A small Zenker’s diverticulum is generally followed, whereas larger and more symptomatic lesions may need surgery. Endoscopic management of a large Zenker’s diverticulum is possible, but is not done in most centres in Canada.
Achalasia can be managed with periodic Botulinum toxin injections to the lower esophageal sphincter, endoscopic balloon dilation or surgery (myotomy). Treatment of Scleroderma esophagus is mainly with high dose proton pump inhibitor. Reflux dysmotility often responds to proton pump inhibitor. Other esophageal dysmotility disorders are sometimes managed with medication such as nitroglycerin or calcium channel blocker. The effect of such treatment is variable. Further extensive detail of these conditions is given in the chapter “Esophagus.”
5. Odynophagia / N. Saloojee
5.1. Description
Odynophagia is pain that is felt while swallowing. This symptom is often present with dysphagia. The pain is generally felt in the retrosternal area. Odynophagia is pain, and should be differentiated from the burning discomfort of heartburn.
5.2 Differential diagnosis
Odynophagia implies a break in the mucosa of the esophagus. In an immunocompromised patient the most common cause is infection. The common infections that cause odynophagia are candida, herpes virus and cytomegalovirus. In an immunocompetent patient, an important cause of odynophagia is pill esophagitis. An ingested pill remains in the esophagus and dissolves there, leading to ulceration. This can be a result of not taking the pill with enough liquid, or lying down too soon after taking the pill. Pill esophagitis is a self-limited condition that resolves without specific therapy. Other less common entities that can cause odynophagia include esophageal cancer, radiation esophagitis, and severe reflux esophagitis. Diagnosis rests on endoscopy and mucosal biopsy. Treatment depends on the underlying condition.
6. Dyspepsia / C. Dubé and N. Saloojee
6.1 Description
The term, “dyspepsia” refers to chronic or recurrent pain or discomfort centred in the upper abdomen. Patients may refer to this as “indigestion”. Various definitions for dyspepsia have been proposed. One such definition is one or more of postprandial fullness, early satiety or epigastric pain. Dyspepsia is a frequent symptom in the general population and, most persons do not seek medical attention.
6.2 Etiology
A variety of conditions can cause dyspepsia. The most common cause is “functional dyspepsia,” also known as “non ulcer dyspepsia.” Functional dyspepsia is the diagnosis in up to 60 % of cases. In such patients, no anatomic or other abnormality can be documented on upper endoscopy (EGD). The pathophysiology of functional dyspepsia is unclear. It may relate to gastric motor dysfunction, visceral hypersensitivity, psychosocial factors or in some cases it may be associated with gastritis due to an infection with Helicobacter pylori.
Peptic ulcer disease and atypical symptoms from gastroesophageal reflux disease
(GERD) are the most common organic explanations for dyspepsia. A less common, not to be overlooked cause of dyspepsia is gastric cancer.
6.3 History and Physical
The approach to a patient with dyspepsia begins with a search for so called alarm symptoms. If present, the possibility of significant pathology increases, and investigation should take place in a timely fashion. Alarm symptoms include unintended weight loss, persistent vomiting, progressive dysphagia, odynophagia, otherwise unexplained anemia, gastrointestinal bleeding, and jaundice. Older age also increases the likelihood that dyspepsia is due to organic pathology. It has been suggested that in Canada, an age greater than 50 years be considered an alarm symptom. If the person with dyspepsia is over the age of 50, or if there are alarm symptoms at any age, EGD needs to be performed promptly.
In a young patient with no alarm symptoms, it is very unlikely that dyspepsia will be due to malignancy.
Numerous other disorders can lead to pain in the epigastrium. Careful history will often allow for identification of these. For example, the pain of biliary colic may be present in the epigastric area, but is often in the right upper quadrant as well. Irritable bowel may cause pain in the upper abdomen, but is associated with altered bowel pattern and relief of pain with defecation. As mentioned before, and to emphasize, be certain to take the appropriate history to exclude ischemic heart disease.
Physical examination is generally unhelpful. Epigastric tenderness is a common, but non-specific finding.
6.4 Investigation and Management
Investigation of dyspepsia generally entails bloodwork. This will reveal if the patient is anemic or has abnormal liver enzymes. Patients with alarm symptoms, over the age of 50 even if there are no alarm symptoms, and patients with persistent dyspepsia despite empiric trials of treatment should undergo endoscopy. If resource availability precludes a timely endoscopy, an upper GI series can be done. If a lesion is seen on the upper GI series, a prompt EGD must be arranged.
In younger patients without alarm features, non-invasive testing for Helicobacter pylori (H. pylori serology or urea breath test) is recommended. If present, H. pylori should be treated. The rationale is that if the patient has an ulcer, treating the infection will eliminate the problem of recurrent ulcers. Also, a minority of patents with functional dyspepsia may improve. In young patients without alarm features, another option is an empiric trial of acid suppressive (proton pump inhibitor) or prokinetic (domperidone) therapy. If these approaches fail and dyspepsia persists, endoscopy can be done.
The results of the treatment of functional dyspepsia is disappointing. Some patients may respond to simple reassurance, dietary manipulation, treatment of H. pylori, trials of proton pump inhibitor, prokinetic or low dose tricyclic antidepressant (eg. amitryptiline 10-25 mg po once nightly).
7. Nausea and Vomiting / M.C. Champion and N. Saloojee
7.1 Description
Nausea is the unpleasant feeling of being about to vomit. Vomiting is the forceful evacuation of stomach contents through the mouth. Vomiting should be differentiated from regurgitation, which is an effortless process. Retching is differentiated from vomiting in that no gastric contents are expelled. Nausea generally precedes vomiting. Vomiting may partially relieve nausea. Vomiting has developed as a defence mechanism, allowing the individual to expel ingested toxins or poisons.
Mechanism ( Figure 1)
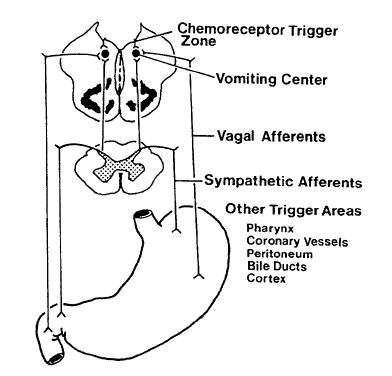
Figure 1. The vomiting center and chemoreceptor trigger zone control vomiting. Peripheral trigger areas send visceral afferent impulses. These excite the vomiting center into action.
Normal motor function of the upper gastrointestinal tract depends on an interplay between the central nervous system and the gut (Figure 1). The neural pathways that mediate nausea are the same as those that mediate vomiting. During nausea, there is gastric relaxation and frequent reflux of proximal duodenal contents into the stomach.
Trigger areas for vomiting exist in the pharynx, cardiac vessels, peritoneum, bile ducts, stomach and cerebral cortex. Excitation of these areas leads to activation of the vomiting centre in the medulla. This is mediated through sympathetic and parasympathetic (vagal) afferents. The chemoreceptor trigger zone exists on the floor of the fourth ventricle on the blood side of the blood-brain barrier. Neurotransmitters, peptides, drugs and toxins may activate the chemoreceptor trigger zone which in turn activates the vomiting centre.
Activation of the vomiting centre leads to forceful abdominal wall contraction, contraction of the pylorus, and relaxation of the lower esophageal sphincter. The glottis closes, and gastric contents are then forcefully expelled.
7.2. History and Differential diagnosis
The differential diagnosis of nausea and vomiting is wide. As alluded to above, nausea and vomiting may be triggered by numerous pathologies arising in many different systems. A good history is therefore critical. Such a history will include inquiry into the timing of the nausea and vomiting. Associated gastrointestinal symptoms such as abdominal pain or diarrhea should be sought. Associated non gastrointestinal symptoms such as headache, chest pain or vertigo are important. As always, obtain the past medical history, past surgical history, family history, and list of medications.
Further questions will suggest themselves as one searches for the specific cause. Table 2 presents a list of more common causes. This list is not exhaustive.
| Table 2. Causes of Nausea and Vomiting | |
| Disorders of the Gut
· Mechanical Obstruction · Gastrointestinal Malignancy · Peptic Ulcer Disease · Cholecystitis · Pancreatitis · Hepatitis · Gastroenteritis · Crohn disease · Functional Gastrointestinal disorders · Chronic Intestinal Pseudoobstruction · Gastroparesis CNS disorders · Migraine · CNS Malignancy · Abscess · Meningitis · Cerebrovascular Accident · Hydrocephalus |
Psychiatric disorders
· Psychogenic vomiting · Anorexia Nervosa · Bulimia · Anxiety / Depression Endocrine causes · Diabetic Ketoacidosis · Hyperthyroidism · Addison’s disease · Hyperparathyroidism · Hypoparathyroidism Cardiac disease · Acute Myocardial Infarction · Congestive Heart Failure Infections · Medications, Drug Withdrawal · Vestibular disorders · Uremia · Pregnancy · Chronic Pain |
Given the wide differential, patients with nausea and vomitting should have a complete physical examination. Attention should be paid to signs of volume depletion, and to clues as to the cause of these symptoms.
7.3. Investigation and Management
Investigations ordered depend on the severity of the nausea and vomiting and whether a specific cause is suggested by clinical evaluation. Potential tests include bloodwork, diagnostic imaging of the abdomen and CNS, and endoscopy.
Management rests on treatment of the underlying disorder and correction of fluid and electrolyte imbalance. A number of medications may be used for their antiemetic action. These include antihistamines such as diphenhydramine, phenothiazines, and gastric prokinetics (domperidone, metoclopramide). Domperidone is generally preferred over metoclopramide as it does not cross the blood-brain barrier and therefore does not cause CNS side effects. Ondansetron is a serotonin antagonist used primarily in chemotherapy-induced nausea and vomiting.
8. Anorexia / M.C. Champion and N. Saloojee
8.1. Description
Anorexia is a loss of, or lack of appetite. Anorexia is a common and non-specific symptoms. It can be a presenting feature of serious pathology such as malignancy. Alternatively, it may arise from a psychologic or functional disorder.
8.2. History and Physical
A thorough history is needed due to the non-specific nature of this symptom. A calorie count may be helpful to assess the actual intake of food. Any weight loss that has occurred should be documented. Physical examination is often normal.
8.3.Differential Diagnosis
Many diseases feature a loss of appetite. These include gastrointestinal pathology, malignancy, chronic renal failure, and congestive heart failure. Psychiatric illnesses such as depression, anxiety, and anorexia nervosa should be considered.
8.4. Approach to Investigation and Management
Choice of investigations for anorexia depends on the severity of illness and whether specific clues are suggested by history and physical. If no organic disease is discovered, psychiatric problems may be present.
9. Gas and Bloating / W.G. Thompson and N. Saloojee
9.1. Description
Patients describing excess gas may be experiencing belching, flatulence, or bloating. Excessive belching or burping is sometimes associated with aerophagia (air swallowing). A degree of aerophagia is physiological, but it may become exaggerated in some patients. Borborygmi is the name given to the noises generated as air and fluid gurgle through the gut. Flatulence is a physiologic phenomenon due to the production of gas by colonic bacteria. Bloating is a perception of abdominal distension. These symptoms often occur together.
9.2. Aerophagia
Commonly, aerophagia is an unwanted but learned habit. Other mechanisms of aerophagia include gum chewing, drinking carbonated drinks, and rapid eating. Stomach gas has the same composition as the atmosphere.
Belching or burping is a physiologic mechanism to relive gastric overdistention with gas. It occurs after a large meal or as a result of aerophagia. Excess belching or burping is rarely a manifestation of significant pathology. It is generally not investigated further unless accompanied by other, more concerning, symptoms.
9.3. Flatulence
Flatulence is a physiologic process. Normally, the gut contains 100 to 200 mL of gas. An average person on a normal diet emits between 500 mL and 1500 mL per day. Excess flatulence is a subjective concern that is defined by the patient’s perception. Hydrogen, carbon dioxide, methane and swallowed nitrogen comprise 99% of colonic gas. The remaining 1% consists of trace gases that often have a strong odour. Such gases include hydrogen sulphide, ammonia, skatole, indole and volatile fatty acids. Most emitted gas originates in the colon. Some carbohydrates such as cellulose are not assimilated in the small intestine. They arrive intact in the colon. Here, resident bacteria digest them to produce hydrogen, carbon dioxide, methane and trace gases. Intestinal microbiotica differs from person to person. Some bacteria produce hydrogen, while others consume it. Differing intestinal organisms can be an explanation for excess flatulence.
Excess flatulence is rarely a sign of serious disease. If symptoms such as weight loss or diarrhea are present, investigations to exclude malabsorption may be undertaken. If a patient is concerned about flatus, treatment may include dietary alteration, simethicone (an agent which causes gas bubbles to break), beano (an agent which absorbs gas), or bismuth. On occasion a trial of antibiotic may be given if small bowel bacterial overgrowth is suspected.
9.4. Bloating
9.4.1. Description
Patients experiencing bloating and distention are often convinced that it is due to excess intestinal gas. Despite visible distention in these patients, abdominal x-rays and computerized tomography (CT) show no increase in bowel gas: gas volume in such individuals is not abnormal. Flatulence may temporarily relieve the perception of bloating.
Gut hypersensitivity may explain the sensation of abdominal bloating. The hypersensitive gut feels full with smaller than usual amounts of gas and fluid and abdominal muscles relax to accommodate the perceived distention. Abdominal girth of female irritable bowel syndrome (IBS) patients complaining of distention may increase 3–4 cm over an eight-hour day. CT has demonstrated increased abdominal girth despite unchanged gas content or distribution. There were no corresponding changes in control subjects.
When patients deliberately protrude their abdomens, the configuration is different from when they are bloated, so a conscious mechanism poorly explains increased abdominal girth.
The reality of the phenomenon of bloating is indisputable, however the mechanism remains a mystery.
9.4.2. Clinical Features
Occasionally, bloating occurs in about 30% of adults and is frequent in 10%. Amongst those with functional disorders such as irritable bowel syndrome or functional dyspepsia, the figures are much higher. Bloating is often the most troublesome symptom of these conditions. Often, the abdomen is flat upon awakening, but distends progressively during the day. The distention often disappears with sleep. Patients complain of the need to loosen their clothing.
Bloating may occur quickly, even over just a minute. It is often aggravated by eating and relieved by lying down. Menstrual periods and stress may worsen bloating in some persons.
9.4.3. Differential Diagnosis
When assessing a patient with bloating and visible abdominal distension, the physician should exclude conditions such as ileus, bowel obstruction, ascites, or intrabdominal tumour. Ileus is a condition in which intestinal motility is reduced. The small and large bowel dilate. This can lead to abdominal distention. Ileus can occur due to medication such as narcotics. It is often seen in the postoperative state. A patient with a bowel obstruction will also have a visibly distended abdomen.
The abdomen can be distended by the presence of fluid in the peritoneal cavity. This condition is called ascites. Rarely, a large tumour in the peritoneal cavity can lead to abdominal distention. Generally these conditions can be separated out with a history and physical as other signs and symptoms are present. If necessary, imaging with abdominal X ray or ultrasound is definitive.
On its own, bloating is not a symptom of organic disease, and should not prompt
investigation.
10. Constipation / C. Dubé, W.G. Thompson and N. Saloojee
10.1. Description
Constipation is also known as obstipation. A precise definition of constipation is elusive due to the variability of what constitutes a normal bowel pattern. Ninety five percent or more of the population have between three movements per day and three movements per week. Some physicians consider that fewer than three movements a week without discomfort or dissatisfaction is normal. Most would agree that hard bowel movements that are difficult to pass constitute constipation even if they occur as often as daily.
The most common terms which patients use to describe constipation are “straining”, hard stools” and the “ inability to have a bowel movement”. Therefore, constipation is a symptom that does not always correlate with infrequent passage of bowel movements.
Constipation is best understood as persistent symptoms of difficult evacuation. This may include straining, stools that are excessively hard, unproductive urges, infrequent bowel movements, or a feeling of incomplete evacuation, often defecation.
10.2. Mechanism
Many conditions can result in constipation. The most common kind of constipation is that associated with irritable bowel syndrome (Table 3). Constipation may be due to primary colonic conditions. Examples are an obstructing colon cancer or idiopathic slow-transit constipation.
Constipation may also be caused by systemic diseases. For example, endocrine disorders (diabetes mellitus, hypothyroidism), metabolic disturbances (hypo- or hypercalcemia), neurologic disorders (multiple sclerosis, Parkinson’s disease), muscular disease (systemic sclerosis, myotonic dystrophy), or medications (opiates, anticholinergic agents).
Proper defecation requires normal transit through the proximal colon, an intact gastrocolic response to a meal, and normal mechanisms of defecation. The gastrocolic response is simply an increase in colonic motility triggered by gastric distention. This reflex is responsible for the urge to defecate after a meal. Defecation requires an intact defecation reflex. In this reflex, stool in the rectum triggers an urge to defecate. Coordinated relaxation of the puborectalis and external anal sphincter muscles must occur.
Table 3. Some causes of chronic constipation
- Primary diseases of the colon
- Stricture
- Cancer
- Anal fissure
- Irritable bowel syndrome
- Idiopathic slow-transit constipation
- Pharmacologic
- Opiates
- Antidepressants
- Anticholinergic agents
- Calcium channel blockers
- Iron
- Laxative abuse
- Pregnancy
- Pelvic floor dyssynergia
- Metabolic disturbances
o Hypercalcemia
o Hypothyroidism
o Diabetes mellitus
- Neurologic and muscular disorders
o Parkinson’s disease o Spinal cord lesion o Multiple sclerosis
o Autonomic neuropathy
o Hirschsprung’s disease
o Systemic sclerosis (Scleroderma)
o Myotonic dystrophy
10.3. Important Points on History and Physical Examination
Taking a good dietary history is important to help manage the person with constipation. This involves an assessment of daily fibre intake, fluid consumption and meal patterns. Many constipated patients do not eat breakfast. As mentioned, colonic motility increases after meals as part of the gastrocolic reflex.
Physical activity stimulates colonic motility. Therefore one should inquire about exercise. Physical impairments leading to impaired mobility will contribute to constipation.
The list of medications should be reviewed. A history of prolonged intake of cathartics, often in the form of herbal remedies or teas, should be sought. Prolonged use of stimulant laxatives can sometimes lead to permanent impairment of colonic motility.
Symptoms such as bloating, abdominal pain relieved with defecation, and alternation of constipation with diarrhea should be sought. These symptoms suggest a diagnosis of irritable bowel syndrome. Stress incontinence suggests a problem with pelvic floor musculature. Weight loss or rectal bleeding raise the possibility of an obstructing colon cancer. Some persons with constipation may leak fluid stool around the inspisated stools, leading to “overslow diarrhea.”
There may be the presence of abdominal distension or palpable stools, but the physical examination of patients with constipation is generally unreavealing. Digital rectal examination (DRE) is useful to identify fissures or hemorrhoids. These may cause constipation, since the patient tries to avoid pain induced by defecation. Fissures or hemorrhoids may also result from constipation. A lax anal sphincter may sometimes suggest a neurologic disorder. The presence of stools in the rectum on DRE may sometimes suggest an impaired defecation reflex.
10.4. Approach to Diagnosis
Constipation is a common symptom. Many patients, particularly those that are younger or those with milder symptoms will need minimal or no investigation.
If investigation is deemed necessary, bloodwork including hemoglobin, inflammatory markers such as erythrocyte sedimentation rate and c reactive protein, blood sugar, thyroid function tests and serum calcium may be done. Inflammatory markers may be elevated in persons with Crohn disease. Crohn’s is an inflammatory bowel disease which may sometimes lead to colonic stricture. Lower endoscopy with either sigmoidoscopy or colonoscopy may be done to rule out structural lesions such as a colonic stricture, malignancy or anal fissure. Endoscopic testing may also detect melanosis coli, a disorder in which there is hyperpigmentation of the colonic mucosa due to chronic use of laxatives. If a patient is over 40 years of age or if alarm symptoms (such as rectal bleeding or weight loss) are present, colonoscopy would be indicated as opposed to sigmoidoscopy. Sigmoidoscopy does not assess the more proximal colon. Alternate tests to assess the structure of the colon include air contrast barium enema or CT colonography. CT colonography is also known as virtual CT and provides radiologic images of the interior of the colon. This test does not allow for biopsy or other intervention, but may be done if colonoscopy cannot be performed or is not readily available.
Occasionally other tests are done. A gut transit study may be revealing. Twenty radiopaque markers are ingested and daily plain abdominal x-rays are taken. If 80% of the markers have disappeared in five days, the transit time is said to be normal. When the transit time is longer than 5 days, the position of the markers may help distinguish slow colonic transit from an anorectal disorder: if remaining markers are seen throughout the colon, slow colonic transit is present. If remaining markers are all in the rectum, an anorectal disorder is present. Anorectal manometry and defecography, are then required.
10.5. Approach to Management
In the majority of patients, a specific disorder is not diagnosed. In these cases, management includes education as to the great variability of bowel habits among the general population. Reassurance is sometimes all that is required.
Where further intervention is needed, dietary changes can be made. This includes the intake of at least three meals a day and adequate amounts of liquids. While no data proves the efficacy of increased fluid intake, 6 to 8 cups per day of water are often recommended. A high fibre intake can be achieved with increased dietary fibre or a commercial fibre product. This increases stool bulk and frequency of bowel movement. The recommended amount of dietary fibre is 20 to 35 g/day. High doses only cause adverse effects, and are not recommended. Regular exercise is often helpful, as it stimulates colonic motility.
Chronic severe constipation may require the use of osmotic agents such as magnesium, lactulose or polyethylene glycol solution. The long-term use of stimulant laxatives such as bisacodyl or senna should be avoided.
More details about this important and common problem are given in the chapter “Colon”.
11. Diarrhea / W.G. Thompson and N. Saloojee
11.1. Description
Diarrhea is defined as bowel movements that are too frequent, too loose or both. Three or more bowel movements per day, or a stool weight of over 200 grams / day is generally considered to be abnormal. In clinical practice, stool weight is rarely measured. Diarrhea is frequently accompanied by urgency. It is important to determine if the patient is using the word “diarrhea” when in fact they have fecal incontinence.
11.2. Mechanism
The four mechanisms of diarrhea are osmotic, secretory, inflammatory and rapid transit. In many instances of diarrhea, two or more of these four mechanisms are at work. Therefore, these mechanisms provide a framework for understanding diarrhea, however they are seldom of great help when approaching a patient in clinical practice. In clinical practice, an anatomical approach is much easier and more useful (please see the chapter on Small Intestine).
If the osmotic pressure of intestinal contents is higher than that of the serum, fluid is drawn into the lumen of the intestinal tract and osmotic diarrhea results. This may result from malabsorption of fat (e.g. celiac disease) or of lactose (e.g. in intestinal lactase deficiency). Certain laxatives, such as lactulose and magnesium hydroxide, exert their cathartic effect largely through osmosis. Certain artificial sweeteners, such as sorbitol and mannitol, have a similar effect. Beware of diabetic candies causing diarrhea. Characteristically, osmotic diarrhea ceases when the patient fasts or sleeps.
Secretory diarrhea occurs when there is a net secretion of water into the intestinal lumen. This may occur with bacterial toxins, such as those produced by E. coli or Vibrio cholerae. It may occur with hormones, such as vasoactive intestinal polypeptide (VIP). Excess VIP is produced by some pancreatic islet cell tumours. In these rare tumours, VIP provokes adenylate cyclase activity in the enterocyte (intestinal epithelial cell). The result is increased cyclic AMP and intestinal secretion. A similar effect may occur as a result of excess bile salts in the colon. Secretory diarrhea does not diminish with fasting, and the patient will be up at night-time to have bowel motion.
Exudative diarrhea results from direct damage to the small or large intestinal mucosa.
This interferes with the absorption of sodium salts and water and is complicated by exudation of serum proteins, blood and pus. Infectious or inflammatory disorders of the gut cause this kind of diarrhea.
Acceleration of intestinal transit may result in diarrhea. An example of this is diarrhea related to hyperthyroidism. The rapid flow impairs the ability of the gut to absorb water, resulting in diarrhea.
11.3. Important Points on History and Physical Exam
The duration of diarrhea is important. If diarrhea has been present for less than two weeks, it is categorized as being acute. Acute diarrhea is almost always due to an infection or food poisoning. Chronic diarrhea, defined as lasting over 2 weeks, has many potential etiologies and often requires investigation.
Knowing the volume of diarrhea can provide a clue to the cause. Distal colonic pathology
generally leads to a small volume diarrhea. Small bowel or proximal colonic pathology generally leads to a large volume diarrhea. Patients may have difficulty in categorizing the volume of diarrhea, and asking them to describe their stool volume as “little squirts” or “big gushes” may be helpful.
Further history includes knowing the characteristics of the diarrhea, such as frequency and consistency. Associated symptoms such as rectal bleeding, weight loss, and abdominal pain should be elicited. Foul smelling, floating or oily stool may indicate fat malabsorption. The presence of intermittent normal or constipated bowel movements suggests irritable bowel syndrome. A thorough medication history is necessary. Recent antibiotic use is of particular importance since this is a risk factor for clostridium difficile, a common cause of diarrhea. Other questions include travel history, exposure to individuals with diarrhea, and sexual practices which might lead to immune deficiency (e.g. HIV/AIDS).
There are many causes of diarrhea, some of which are summarized in Table 4. This list is not exhaustive. Each of these causes will suggest further questions to the interviewer. Physical exam is generally more useful in assessing the severity of diarrhea, rather than finding a cause. Volume status is best determined by looking for changes in pulse and blood pressure.
| Table 4. Differential Diagnosis of Diarrhea | |
| Ø Acute Diarrhea | |
| o Infection
o Food Poisoning |
o Initial Presentation of Chronic Diarrhea |
| Ø Chronic Diarrhea | |
| Ø Gastric
− Dumping syndrome Ø Small intestine − Celiac disease − Lymphoma − Whipple’s disease − Parasitic infection ( ex Giardia lamblia) − Bacterial overgrowth − Bile salt malabsorption − Diabetic Autonomic Neuropathy − Short Bowel Syndrome Ø Large bowel − Villous adenoma / Colon cancer − Inflammatory bowel disease (ulcerative colitis, Crohn disease) − Irritable bowel − Functional diarrhea – HIV related infections |
Ø Pancreas
− Chronic pancreatitis − Islet cell tumours (e.g. VIPoma) Ø Drugs − Antibiotics − Alcohol − Laxatives − Nonsteroidal anti-inflammatories − Sorbitol, fructose − Many others Ø Metabolic/Endocrine − Hyperthyroidism − Addison’s disease − Diabetes − Carcinoid syndrome |
11.4. Investigation and Management
Acute diarrhea is self-limiting and may not need investigation. If it is more severe, investigation focuses on searching for an infection through stool tests for culture and sensitivity, ova and parasites and Clostridium difficiletoxin. Viral studies are important in infants.
As noted, the differential diagnosis of chronic diarrhea is long. Testing will vary depending on the individual case, case, but may include bloodwork, stool testing, gastroscopy and small bowel biopsy, colonoscopy and biopsy, and imaging of the small bowel and abdomen.
Practice points
- Gastrointestinal complaints are common in the general population
- Fear of underlying malignancy is a common reason for a complaint to come to medical
attention
- Functional GI disorders are common
- Thorough and careful history-taking is crucial in gastroenterology. This includes family
history for such disorders as malignancy, celiac disease, IBD, and liver disease
- Alarm symptoms such as weight loss, bleeding, dysphagia should lead to prompt investigation for organic pathology
- The history may lead to a differential diagnosis (increasing pre-test probability of a physical finding), so that additional care and the use of supplemental tests will be used on physical examination
- Physical examination involves much more than just the abdome It should include a thorough examination of all parts of the body, looking for extraintestinal signs such as may occur in persons with cirrhosis, celiac disease, inflammatory bowel disease, and nutritional deficiencies
12. Malnutrition / D.G. Patel
12.1 Description
Nutrition may be defined as the process by which an organism utilizes food. This complex process involves ingestion, digestion, absorption, transport, utilization and excretion. Any alteration in one or many of these factors can produce malnutrition. Globally, primary malnutrition due to lack of food is the most common cause. Malnutrition in a developed country such as Canada may be due to inadequate intake of nutrients, malabsorption and/or the hypercatabolism accompanying a critical illness. Protein-energy malnutrition is increasingly recognized in eating disorders such as anorexia nervosa.
12.2 Mechanism
More common reasons for malnutrition include the following.
- Lack of food intake due to anorexia, depression or symptoms exacerbated by food intake (dysphagia, odynophagia, nausea, vomiting or abdominal pain)
- Maldigesti Examples include pancreatic disease and bile salt deficiency due to cholestatic hepatobiliary disease or ileal disease
- Malabsorpti For example, mucosal disease of the small intestine or loss of intestinal surface area due to resection
- Excessive loss of nutrient For example, protein-losing enteropathy seen in many intestinal disorders
- Medicati For example, cholestyramine used for bile salt induced diarrhea can worsen steatorrhea in the case of an extensive ileal resection
- Alcoholi Alcoholics rarely consume a well-balanced diet. They depend heavily on “empty” calories from alcohol. Protein and vitamin deficiencies, particularly of the B- complex group, are extremely common. Alcohol is a toxic agent that even in the presence of adequate nutritional intake can produce damage to the pancreas, liver and small bowel mucosa, aggravating malnutrition
12.3 Signs of Malnutrition
- Weight loss
- Muscle wasting. Particularly evident in the temporal area and dorsum of the hand between the thumb and index finger. It suggests protein-calorie deficiency Signs of fat soluble vitamin (ADEK) deficiency. Decreased visual acuity, low bone mass, and easy bruising
- Cheilitis. Fissures at corners of mouth due to riboflavin (B2), iron deficiency.
- Glossitis. Due to B12, folate or iron deficiency
- Hepatomegaly. Fatty liver is a common finding in protein malnutrition or alcoholism.
- Peripheral neuropathy. Decreased position sense, decreased vibration sense or ataxia may result from B12 deficiency
- Anemia. Can be due to iron, folate or B12 deficiency. Can be due to anemia of chronic disease
- Peripheral edema (Hypoalbuminemia)
13. Acute Abdominal Pain / J.M. Watters and N. Saloojee
13.1 Description
Acute abdominal pain refers to pain that has been present for a short period of time, generally less than 24 hours. The term acute abdomen is best used to describe abdominal pain severe enough to suggest a serious intraabdominal condition. Although not entirely accurate, acute abdomen is sometimes used synonymously with peritonitis (peritoneal inflammation). Since some patients with an acute abdomen require resuscitation and early surgical treatment, it is important to assess the patient and establish a plan of management as soon as possible. The initial goal if the patient has an acute abdomen is not necessarily to make a definitive diagnosis, but rather to identify if the patient requires prompt surgical intervention.
13.2 Mechanism
Acute abdominal pain may be referred to the abdominal wall from intraabdominal organs (visceral pain) or may involve direct stimulation of the somatic nerves in the abdominal wall (somatic pain). Peritonitis results in somatic pain.
Visceral pain arises from such things as tension in the bowel wall (e.g., distension or vigorous contraction), mesenteric traction, or irritation of the mucosa or serosa of the bowel (e.g., chemical irritation, bacterial contamination, ischemia). Foregut pain is typically epigastric in location, midgut pain is central, and hindgut pain is felt in the lower abdomen. Organs that are bilateral give rise to visceral pain that is predominantly felt on one or the other side of the body.
Somatic pain is more precise in location than visceral pain. That is the main difference between the two. Somatic pain corresponds more directly to the anatomic site of the underlying pathology. Somatic pain occurs with stimulation of pain receptors in the peritoneum and abdominal wall.
Occasionally, pain is referred to the abdomen from extra-abdominal sites (e.g. lower lobe pneumonia). Unusually, acute abdominal pain is a feature of systemic disease (e.g. diabetic ketoacidosis).
13.3 History
The history should focus on the chronology, location, intensity and character of the pain. Aggravating and relieving factors should be sought. As always, inquire about associated symptoms, past medical and surgical history, medications, family and social history (including smoking, alcohol and substance abuse).
Severe pain of sudden onset may suggest a catastrophic event (e.g. perforation of an ulcer, intestinal ischemia, or rupture of an aortic aneurysm). Pain that occurs episodically is sometimes referred to as “colic”. Colicky pain corresponds to peristaltic waves. It eases or disappears between waves. One example is the intermittent, mid-abdominal pain of uncomplicated small bowel obstruction. Another is the intermittent flank pain radiating anteriorly to the groin that accompanying ureteric obstruction from a renal stone. Biliary “colic” is a misnomer, in that biliary pain is typically steady. It is usually felt in the epigastrium or right upper quadrant.
As mentioned visceral pain is poorly localized as compared to somatic pain. Nevertheless, the initial location of pain can provide a clue as to the origin. Also, radiation of pain may provide important clues to diagnosis. Irritation of the diaphragm, from peritonitis, for example, may cause shoulder tip pain. Biliary tract pain may radiate to the right scapular region. Pain arising from retroperitoneal structures may be perceived in the back (e.g. pancreatitis, leaking abdominal aortic aneurysm).
The character and subsequent evolution of acute abdominal pain may give a clue as to the site and nature of the underlying pathology. For example, pain with movement (e.g. riding in a car or walking) suggests the presence of peritonitis.
13.4 Associated Symptoms
Anorexia, nausea and vomiting are more common in diseases of the gastrointestinal tract but are not specific to a particular disorder. Abdominal distention and obstipation may suggest intestinal obstruction. Bloody diarrhea may arise from severely inflamed, ulcerated or infarcted bowel. Jaundice points to a hepatobiliary problem. In women, an accurate menstrual history is important. Urinary symptoms may suggest a genitourinary diagnosis (e.g. pyelonephritis, renal stones).
13.5 Physical Examination
In some patients with acute and severe abdominal pain, analgesics are delayed until after the physical exam. This generally applies to a subset of such patients presenting for evaluation in the emergency department. Analgesia may impair the sensitivity of physical examination when signs are subtle. Medication should be given promptly once the assessment has been completed. It should be given if the physical exam will be unavoidably delayed.
The physical exam begins with an evaluation of blood pressure, pulse and respiratory
rate. Abdominal pathology may lead to systemic effects such as hypotension, tachycardia, or tachypnea. A careful physical examination will also identify pertinent extra-abdominal findings such as jaundice or lymphadenopathy.
Examination of the abdomen is performed with the patient supine. The steps are inspection, auscultation, palpation and percussion. Inspection of the abdomen should note any distention, mass, hernia, or scar. Of note is that the patient with peritonitis typically lies immobile. This is because any movement increases peritoneal irritation and pain.
Unlike examination of other systems, auscultation is often performed before palpation. Palpation can stimulate intestinal peristalsis and alter the result of auscultation. Auscultation may reveal a range of bowel sounds. A silent abdomen indicates an ileus or lack of intestinal peristalsis. Causes of ileus include the postoperative state, medications such as narcotics, or peritonitis. Hyperactive bowel sounds may be heard when a bowel obstruction is present. Bruits are sounds created by turbulent flow through a stenotic artery. When present, they suggest vascular disease.
Gentleness is the key to abdominal palpation. Palpation detects and localizes tenderness, muscle guarding, rigidity and masses. Palpation should begin in an area away from where pain is experienced, progressing to the area of pain last. Involuntary guarding and rebound tenderness are signs of peritonitis. Guarding refers to contraction of abdominal wall muscles when the abdomen is palpated. Guarding is only important if muscle contraction is involuntary. Involuntary guarding occurs as a protective mechanism when peritoneal inflammation (peritonitis) is present. Voluntary guarding occurs when a patient tenses abdominal wall muscles in response to that abdominal wall pressure. It is a meaningless finding that is commonly seen. Guarding may be localized (e.g. uncomplicated appendicitis) or generalized throughout the abdomen (e.g. perforated appendicitis with diffuse contamination of the peritoneal cavity). In some instances of peritonitis, the muscles are in a state of continuous contraction. They are rigid or “board-like”, even without palpation. In subtle situations, peritonitis is suggested by the triggering of pain in the area of suspected pathology (e.g., appendicitis) through palpation elsewhere on the abdominal wall, by having the patient cough or by gently shaking the pelvis.
Gentle percussion is also a very useful way to assess peritoneal irritation, as well as to assess the nature of abdominal distention. Rebound tenderness, another sign of peritonitis, is elicited by deeply palpating the area of concern and then suddenly releasing the abdominal wall. Severe pain felt on release of the abdominal wall is rebound tenderness. This manoeuvre can be very distressing to the patient with peritonitis, so it is often not done. Rectal and pelvic examinations should be carried out and recorded by at least one examiner. The sites for inguinal and femoral hernias should be specifically examined. Femoral pulses should be palpated.
13.6 Differential Diagnosis
The list of causes of abdominal pain is long. Table 5 provides a list of common or important conditions to consider. The list is not meant to be complete. Intra-abdominal conditions requiring surgery (open or laparoscopic) are the most common causes of an acute abdomen. Some conditions require immediate surgery (e.g. ruptured abdominal aneurysm). They must always be included in the differential diagnosis, therefore, and confirmed or excluded promptly. In other instances, the specific diagnosis and the need for surgery may take some time to establish. The likelihood of specific diagnoses varies to an extent with the age of the patient. Clinical presentations are more likely to be atypical in the elderly and in patients with coexisting conditions (such as diabetes or stroke). Particular care must be taken to not overlook an important intra-abdominal process in such patients.
One must always consider in the differential diagnosis: (1) intra-abdominal conditions for which surgery is not indicated (e.g. acute pancreatitis, (2) extra-abdominal (e.g. pneumonia) or systemic conditions (e.g. diabetic ketoacidosis) that can be accompanied by acute abdominal pain.
Table 5. Differential Diagnosis of Acute Abdominal Pain
- Peptic Ulcer Disease
- Mesenteric ischemia/infarction
- Gastroenteritis
- Cholecystitis
- Pancreatitis
- Appendicitis
- Functional Conditions ( eg. irritable
bowel syndrome, non ulcer
dyspepsia )
- Inflammatory Bowel Disease
- Bowel obstruction
- Diverticulitis
- Ruptured Abdominal Aortic Aneurysm
- Incarcerated hernia
- Hepatitis
- Pyelonephritis / Cystitis
- Gynaecologic conditions (ex. pelvic
inflammatory disease, ruptured ectopic
pregnancy)
- Extra abdominal and Systemic Causes
13.7 Investigations
In many instances, a careful history and physical examination provide the clinical diagnosis. Complete blood count (CBC), serum amylase or lipase, electrolytes, creatinine, liver enzymes, glucose and urinalysis are routine. Other blood work is obtained as indicated. Pregnancy testing should be done when appropriate. Chest and plain abdominal x-rays are obtained routinely unless the diagnosis is clear (e.g. appendicitis). The presence of free peritoneal air indicates a perforated viscus. Abdominal x-rays can also provide information about the pattern of bowel gas (e.g. intestinal obstruction), edema and pneumatosis of the bowel wall, retroperitoneal structures (e.g. pancreatic calcification), and bony structures (e.g. fractures, bone metastases).
More sophisticated diagnostic imaging is often valuable. Ultrasound is very useful in the diagnosis of biliary tract disease (gallstones), abdominal aortic aneurysm, gynecologic disease and is often used in suspected appendicitis. Increasingly, abdominal CT scanning is being used, often obviating the need for more invasive or uncomfortable studies.
Other imaging modalities that may be ordered depending on the case include intravenous pyelography to assess the genitourinary tract or mesenteric angiography. The choice of investigation should be discussed with a radiologist.
Endoscopy (gastroscopy or colonoscopy) may be indicated in some cases. Laparoscopy has an important diagnostic role, as well as allowing definitive surgical therapy (e.g. appendectomy, omental patch of a perforated duodenal ulcer).
13.8 Approach to Management
A reasonably specific diagnosis or focused differential can usually be established early on. This is the basis for determining further management. In some instances (e.g. possible appendicitis), careful observation with repeated examination and selected imaging studies (e.g., ultrasound) allow a diagnosis to be reached. In some individuals, acute abdominal pain of mild to moderate severity resolves without a confirmed diagnosis. In patients with more serious conditions, intravenous fluid administration, other supportive measures and monitoring must be instituted following rapid initial assessment, even before a specific diagnosis can be made. In such individuals, diagnostic and therapeutic manoeuvres must proceed in a coordinated and efficient manner. Occasionally, patients with an acute abdomen, typically those who are unstable despite resuscitation or who have obvious generalized peritonitis, require urgent CT scan or ultrasound of the abdomen, or even laparotomy without a definitive preoperative diagnosis.
14. Chronic Abdominal Pain / W.G. Thompson and N. Saloojee
14.1. Description
As discussed below, there is a wide differential to chronic abdominal pain. In many cases however, no objective abnormality can be found. Such patients are said to have functional abdominal pain. Ten percent of children suffer recurrent abdominal pain and approximately 20% of adults have abdominal pain at least six times per year unrelated to menstruation. Functional abdominal pain syndrome is formally described as pain present continuously or near continuously for 6 months or more in which there is no relationship of the pain to eating, defecation, menses and in which no organic pathology can be found. Patients not strictly meeting this duration of pain may still be said to have functional abdominal pain.
14.2. Mechanisms and Causes
Functional abdominal pain is regarded is as being related to dysfunction of the brain-gut axis: pain is perceived in the abdominal region in the absence of pathology. The central nervous system and psychosocial stressors combine to lead to a heightened experience of pain.
Of course, chronic abdominal pain may be caused by many organic diseases. The pain of peptic ulcer disease may be food related and may improve with antacid. Most ulcers are related to Non Steroidal Anti inflammatory Drugs (NSAID) or H. pylori infection.
Intermittent obstruction of the cystic duct by a gallstone is known as biliary colic.
Characteristically, patients experience significant episodic right upper quadrant (RUQ) and sometimes epigastric pain after meals. The pain may last hours in duration. However it subsides spontaneously and the patient is systemically well. Cholecystitis refers to a more long lasting, continuous pain in the same area due to impaction of a stone in the cystic duct. Subsequent dilation and inflammation of the gallbladder occurs. Such patients may have fever or be systemically unwell. Obstruction of the common bile duct with a stone (choledocholithiasis) results in pain and jaundice. The presence of fever in such a patient indicates infection due to stasis of material in the biliary tree (cholangitis).
Other causes of chronic or recurrent abdominal pain include chronic pancreatitis, intra abdominal neoplasms, inflammatory bowel disease, mesenteric ischemia, partial bowel obstruction, adhesions, renal colic and gynaecologic disorders.
As mentioned, functional abdominal pain is unrelated to eating, defecation or menses. Irritable bowel syndrome, is an almost identical disorder but is distinguished by disordered defecation. Functional abdominal pain may be due to a normal perception of abnormal gut motility or an abnormal perception of normal gut motility. It may not be due to the gut at all in that patients frequently have accompanying psychosocial difficulties.
14.3. Important Historical Points and Physical Examination Features
When chronic abdominal pain relates to a bodily function (defecation, eating, micturition or menstruation) investigation should focus upon the involved system.
Functional pain is more frequent in those who have had recent conflicts, have experienced a death in the family, or have become overly concerned with fatal illness. There may be a history of traumatic life events. Depression and anxiety are frequent. Patients with functional abdominal pain do not have alarm symptoms such as fever, weight loss, or rectal bleeding. Physical examination and lab tests are normal.
14.4. Diagnosis and Management
Diagnostic testing for chronic abdominal pain is similar to that for acute abdominal pain. Investigation involves a combination of bloodwork, urinalysis, diagnostic imaging and endoscopic testing. Management of organic causes of the chronic abdominal pain is directed at the underlying disease process.
For patients with functional abdominal pain, the physician’s responsibility is to reassure the patient that no serious disease exists. A strong patient-physician relationship is needed. Where such a relationship does not exist, the patient may consult many doctors without satisfaction. The focus is to help the patient manage the pain, rather than cure it. It is important to investigate to a degree to reassure both patient and physician that the diagnosis is correct. However, it is also important not to continually repeat investigations in the belief something is being missed. Drugs, especially narcotics, should be used with restraint. Some individuals benefit from low-dose antidepressants, as in other chronic pain syndromes. These patients test our skill in the art rather than the science of medicine.
15. Jaundice / L.J. Scully and N. Saloojee
15.1. Description
A state characterized by increased serum bilirubin levels (hyperbilirubinemia) and a yellow appearance due to deposition of bile pigment in the skin and mucus membranes.
15.2. Mechanism
Bilirubin is a waste product of hemoglobin metabolism. Interruption of the breakdown pathway at any of a number of steps, or a marked increase in load due to red blood cell destruction, results in an increase in serum bilirubin and if high enough, clinical jaundice. Under normal circumstances, senescent red blood cells are taken up and destroyed in the reticuloendothelial system. Through a number of steps the heme molecule of hemoglobin is converted to bilirubin which is, tightly bound to albumin, and transported in the plasma to the liver cells. Hepatocytes take up bilirubin, conjugate it to glucuronide and excrete the bilirubin diglucuronide in bile into the duodenum. In the bowel, bacteria break down bilirubin to urobilinogen, 80% of which is excreted in the feces, contributing to the normal stool colour. The remaining 20% of urobilinogen is reabsorbed and excreted in bile and urine (enterohepatic circulation of urobilinogen).
Functional defects in bilirubin metabolism or anatomic obstruction to excretion into the biliary system will result in an increase in serum bilirubin and jaundice. A large increase in the breakdown products of hemoglobin alone (e.g. hemolytic anemia) will cause an increase in serum unconjugated bilirubin. If the problem lies after the uptake and conjugation step, the increase is in serum conjugated bilirubin. In adults, aside from hemolysis or the common benign unconjugated hyperbilirubinemia of Gilbert’s syndrome, most patients with jaundice have a conjugated hyperbilirubinemia. Causes of jaundice are usually classified as: (1) hemolysis; (2) genetic defects in bilirubin handling; (3) hepatocellular disease; and (4) obstruction or cholestasis.
15.3. Clinical Presentation
Clinical jaundice is detected when the serum bilirubin level reaches 2–4 mg/dL (40–80 μmol/L). Jaundice is usually preceded by a few days of pale stools (as excretion of bilirubin into the intestine is decreased) and dark urine (due to increased glomerular filtration of conjugated bilirubin). Jaundice is usually first detected in the sclera, although the bilirubin is actually deposited in the overlying conjunctival membranes. Yellow skin without scleral icterus should suggest carotenemia (excess intake of foods high in carotene) or the ingestion of such drugs as quinacrine.
Patients with jaundice due to a hepatocellular cause (e.g. viral hepatitis) often have nausea, anorexia, hepatomegaly and right upper quadrant discomfort. Patients with jaundice due to a cholestasis often experience pruritis, presumably from deposition of bile salts in the skin. Physical exam may reveal an abdominal mass such as a dilated gallbladder. Other historical points to ask include inquiring about viral hepatitis risk factors (e.g. IV drug use, prior transfusions), history of alcohol abuse, medications, and family history of liver disease.
The end stage of liver disease of any cause is cirrhosis of the liver. Such patients have a small, shrunken and nodular liver. Signs or so-called stigmata of chronic liver disease may be found. These include spider nevi, gynecomastia, palmar erythema, Dupuytrens contracture, signs of portal hypertension (ascites, splenomegaly, dilated periumbilical veins) and asterixis (flapping of the outstretched hands, a sign of hepatic encephalopathy).
15.4. Approach to Diagnosis
Initially, the evaluation of jaundice is to determine whether it is primarily due to conjugated or unconjugated hyperbilirubinemia (Figure 2). Serum bilirubin can be fractionated from total bilirubin into conjugated and unconjugated. The presence of bile in the urine determined by a test strip at the bedside confirms that the bilirubin rise is predominantly in the conjugated form. If the bilirubin is primarily unconjugated, hemolysis or genetic defects are implicated. A blood smear showing schistocytes or fragmented red calls confirms hemolysis. In adults, Gilberts syndrome is an inherited genetic disorder of impaired bilirubin conjugation. Particularly at times of physiologic stress, a mild unconjugated hyperbilirubinemia may occur. The disorder is innocuous and follows a benign natural history.
If the hyperbilirubinemia is conjugated, liver enzyme tests (AST, ALT, GGT and alkaline phosphatase) will help determine if the jaundice is primarily due to hepatocellular damage (high AST and ALT) or obstruction/cholestasis (high GGT and alkaline phosphatase). Transaminases (AST/ALT) are released by damaged hepatocytes. Also check the patients INR and albumin concentration, as these are markers of synthetic function of the liver and reflect a more chronic underlying disease process.
Cholestatic jaundice requires an ultrasound as the best, first test. Dilation of intra- and/or extrahepatic bile ducts indicates an anatomic problem. In this case, the cholestasis is caused by an extrahepatic problem (e.g. pancreatic neoplasm compressing common bile duct or stones in the common bile duct). Lack of biliary dilation indicates intrahepatic cholestasis ( e.g. medication adverse effect).
Further tests such as viral serology, markers for other liver diseases, further liver imaging, endoscopic retrograde cholangiopancreatography (ERCP), MRI of the biliary area (MRCP), CT scan of the abdomen, and liver biopsy are ordered as needed.
Figure 2. Approach to hyperbilirubinemia.
15.5. Management
Management of the specific disorders causing jaundice is contained in the chapters on the hepatobiliary and pancreatic systems. In general, hepatotoxins (e.g. alcohol, medication) should be withdrawn, biliary obstruction should be relieved if present, and therapy for the underlying disorder instituted where possible.
16. Ascites in Chronic Liver Disease / L.J. Scully and N.Saloojee
16.1. Definition
Ascites is the accumulation of free fluid in the peritoneal cavity.
16.2. Mechanisms
With significant liver disease (cirrhosis), ascites is a result of activation of the renin- angiotensin-aldosterone system and portal hypertension. Renin-angiotension-aldosterone activation leads to sodium and water retention. Increased portal pressure leads to transudation of fluid from the capillaries in the portal system to the peritoneal cavity. Ascites may also occur in patients without liver disease. Intra-abdominal malignancy or chronic peritoneal infection (e.g. tuberculosis) can lead to ascites as a result of protein rich fluid being actively secreted.
16.3. Signs and Symptoms
Ascites most commonly presents with increasing abdominal girth. There is often an uncomfortable feeling of distention. Sometimes, there is nausea and anorexia. Diaphragmatic elevation or a pleural effusion (ascites fluid tracking into pleural space) can lead to shortness of breath. Ankle edema may accompany ascites. Clinical examination reveals a distended abdomen and bulging flanks on inspection. “Shifting dullness” or a “fluid thrill” may be elicited. Smaller amounts of fluid may be detected on ultrasound when clinical signs are absent. One should look for other signs of portal hypertension, such as dilated abdominal wall veins or splenomegaly.
16.4. Differential Diagnosis
Newly developed ascites must have a diagnostic aspiration (paracentesis) to determine the albumin level, cell count and cytology. The fluid should be clear and straw coloured. If the fluid is bloody, chronic infection (e.g. tuberculosis) or malignancy should be sought. Determining the etiology of ascites hinges on the serum ascites albumin gradient (SAAG). The gradient is calculated by subtracting the ascites albumin from the serum albumin. If the gradient is high (>11 g/L), then the ascites is due to portal hypertension. If the gradient is low (>11 g/L), then the ascites is not from portal hypertension. The likeliest cause of low gradient ascites is malignancy. A low gradient results from ascites that is high in protein, so that the ascites albumin level is close to that of the serum.
Ascitic fluid may become infected, in which case the white blood cell count will be elevated (>250 neutrophils/uL) in the fluid. Bacterial infection of ascites fluid can be due to a perforation in the GI tract (e.g. perforated appendix) or it can be a spontaneous occurrence (spontaneous bacterial peritonitis).
16.5. Approach to Management
Management of ascites begins with salt restriction. Most cases also require addition of a diuretic such as spironolactone and/or furosemide. If ascitic fluid reaccumulates despite these measures, aspiration of large quantities of ascites fluid or large volume paracentesis may be
necessary. Six to eight litres can be safely removed at a time. If ascites remains uncontrolled, options include repeated large volume paracentesis, transjugular intrahepatic portosystemic stent shunt (TIPS) or liver transplant.
17. Gastrointestinal Bleeding / A. Rostom, C. Dubé and N.Saloojee
17.1. Description
Gastrointestinal (GI) bleeding may be referred to as upper, lower, obscure or occult. Upper GI bleeding commonly presents with hematemesis (vomiting of red blood), coffee-ground emesis and/or melena (black, tarry stools). The black colour of melena is the result of degradation of blood by intestinal bacteria. In comparison, hematochezia (bright red or maroon coloured blood per rectum) is usually a sign of lower GI bleeding. It is important to note that a very brisk upper GI bleed can lead to hematochezia as blood passes rapidly through the gut and is not degraded. Upper GI bleeding occurs proximal to the ligament of Treitz. Small bowel bleeding occurs from the ligament of Treitz to the distal ileum. Lower GI bleeding occurs from the terminal ileum and colon.
Occult bleeding is bleeding that is not apparent to the patient. The quantity of bleeding is small, so that the colour of stools is not altered. Patients may present with a positive fecal occult blood test (FOBT) result and/or iron-deficiency anemia (IDA). Chronic loss of small amounts of blood can eventually lead to significant IDA. Obscure bleeding is defined as bleeding of unknown origin that persists or recurs after negative initial endoscopies (colonoscopy and upper endoscopy). Most commonly, the source of obscure bleeding is the small bowel. Obscure bleeding may be overt (i.e., hematemesis, melena or hematochezia), or may be occult such as persistent IDA. The important causes of upper and lower GI bleeding are presented in Tables 3 and 4 respectively.
| Table 6. Causes of Upper GI Bleeding | |
| Common | Less Common |
| o Peptic Ulcer Disease : Gastric Ulcer, Duodenal Ulcer
o Esophageal Varices o Mallory-Weiss Tear o Neoplasm : Esophageal cancer, Gastric Cancer, Lymphoma |
o Esophagitis
o Portal Hypertensive Gastropathy o Vascular : angiodysplasia, gastric antral vascular ectasia ( GAVE; “watermelon” stomach), Dieulafoy lesion) o Aortoenteric Fistula o Hemobilia o Crohn disease |
| Table 7. Causes of Lower GI Bleeding | |
| Common | Less Common |
| o Diverticular bleed
o Angiodysplasia o Colon Cancer o Ischemic Colitis o Inflammatory Bowel Disease |
o Radiation Proctitis
o Post-polypectomy bleeding |
17.2. Approach to Diagnosis and Management
The initial evaluation of the patient with acute GI bleeding involves assessment of the “ABCs” (Airway, Breathing, and Circulation). Patients with upper GI bleeding are at risk of airway compromise from aspiration of vomited blood. Another risk factor for some patients is a reduced level of consciousness due to shock or hepatic encephalopathy. Some patients may require supplemental oxygen or even intubation for airway protection and/or assisted breathing. During the assessment of the hemodynamic status, IV access is crucial. In a significant GI bleed, two large bore peripheral IVs (18 gauge or greater) are placed for fluid and blood product restoration. At this stage, blood should be drawn for typing and cross-matching. Bloodwork should include CBC, INR, electrolytes, urea, creatinine, as well as albumin and liver enzymes.
It is important to remember that, hemoglobin (Hb) and hematocrit (Hct) may not be low at presentation. These measures reflect the red blood cell concentration. Over the ensuing 36–48 hours, most of the volume deficit will be repaired by the movement of fluid from the extravascular into the intravascular space. Only at these later times will the Hb and Hct reflect the true degree of blood loss. Furthermore, if a patient presents with an acute GI bleed and the initial Hb is low, one should expect the Hb to continue to decline and so transfusion should be considered. Some patients, in particular those with GI malignancies, may have had chronic occult bleeding prior to their acute presentation. The result is hypochromia and microcytosis from iron deficiency. Coagulopathy, due to medications (e.g. warfarin) or liver dysfunction, should be corrected. An elevated blood urea nitrogen (BUN) value in the presence of a normal creatinine may be a sign of upper GI bleeding. The elevated BUN is due to blood being absorbed from the proximal small bowel.
Pharmacotherapy for GI bleeding includes intravenous proton pump inhibitors in cases of suspected bleeding from peptic ulcer disease and intravenous administration of somatostatin analogs (octreotide) in suspected cases of esophageal variceal bleeding. Because of the seriousness of upper GI bleeding, and the not-uncommon delay in obtaining in endoscopy for purposes of diagnosis and endoscopic hemostatic therapy, pharmacotherpy is often given on initial presentation, before the cause of bleeding has been definitively determined.
In acute GI bleeding, symptoms associated with blood loss include weakness,
diaphoresis, pre-syncope, and syncope. Patients with a chronic, slow bleed may present with iron deficiency anemia. Signs and symptoms include pallor, fatigue, and dyspnea. In a predisposed individual, anemia can lead to congestive heart failure or angina. In all cases of GI bleeding, information should be gathered about medication use, in particular the intake of NSAIDs, Aspirin (ASA) or anticoagulants. Other important data includes a prior history of peptic ulcer disease, history of abdominal surgery (e.g. vascular grafts raise the suspicion of aorto-enteric fistulas), and a history of chronic liver disease or alcohol abuse. Look for signs of chronic liver disease on physical examination. The hemodynamic status should be interpreted in light of the patient’s abilities to compensate for hypovolemia. In a young and fit adult, the presence of a resting or orthostatic tachycardia should be interpreted as a sign of significant volume loss, while the loss of an equivalent blood volume in an elderly or debilitated subject would more likely be manifested by hypotension or shock.
Once supportive measures have been undertaken, the patient should be assessed with a view towards identifying the source of bleeding (ie. upper or lower). Bright red emesis is suggestive of bleeding from esophageal varices or of a brisk upper GI source. In a duodenal bleed, blood may or may not reflux into the stomach. Therefore, the absence of hematemesis, coffee ground emesis or a bloody aspirate from nasogastric suction does not rule out an upper GI bleed. The pigmentation of the stool will depend on the length of time in transit along the bowel. In an upper GI bleed, transit time is usually longer than a lower bleed. Therefore, an upper GI or proximal small bowel bleed usually leads to melena. Conversely, a lower GI bleed generally leads to hematochezia. In determining the likely source of bleeding, the clinician needs to interpret the patient’s manifestations of bleeding in conjunction with the hemodynamic status. Blood originating from the left colon typically is bright red. However, hematochezia associated with hemodynamic instability raises the suspicion of a brisk upper GI bleed. Similarly, while the passage of melena is most commonly associated with an upper GI source, dark burgundy or black stools can sometimes be encountered in proximal colonic bleeds. In the absence of spontaneous passage of stools, a digital rectal examination to determine the stool color will be most informative.
Under certain circumstances it may be difficult to determine if the GI bleed, particularly if it is significant, is of upper or lower origin. It is then safest to proceed on the assumption of an upper GI bleed, and to arrange for early upper endoscopy. Many causes of upper GI bleeding are amenable to endoscopic therapy. If the bleed is due to a peptic ulcer, upper endoscopy allows stratification of rebleed risk based on the appearance of the ulcer. In a suspected upper GI bleed, the timing of upper endoscopy varies. Early upper endoscopy is done if there are signs of a brisk bleed, a variceal bleed is suspected, the patient is older or has numerous comorbidities.
Most lower GI bleeds stop spontaneously, so the treatment is mainly supportive. If a lower GI bleed does not stop, angiography should be pursued to localize the source of bleeding and possibly embolize the source. If these measures fail to stop a lower GI bleed, surgery may become necessary. Colonoscopy has little utility in an active lower GI bleed since blood obscures visualization. The role of colonoscopy in lower GI bleeding is mainly diagnostic, once bleeding has slowed and bowel cleansing can be achieved.
In the case of a GI bleed where upper endoscopy and colonoscopy are negative, small bowel investigations may become necessary. Radiologic options include a small bowel follow through or CT enterography (protocol to look at small bowel). Wireless capsule endoscopy involves ingestion of a pill sized camera to take pictures of the small bowel. Enteroscopy involves a long scope inserted from the mouth to examine the proximal small bowel. Balloon enteroscopy is a newer endoscopic technique in which total endoscopic examination of the small bowel is possible.
18. Abdominal Mass / S. Grégoire and N. Saloojee
18.1. Description
When an abdominal mass is discovered on physical examination, one must define its nature. Using a systematic approach often permits the identification of the mass before the use of sophisticated tests.
18.2. Important Points in History and Physical Examination
Important clues in the history and general physical examination may help to identify the enlarged viscus. For example, in a young patient presenting with diarrhea, weight loss and abdominal pain, finding a right lower quadrant mass would suggest inflammatory bowel disease. However, an abdominal mass may be discovered during physical examination of an asymptomatic individual. Certain observations made during the abdominal examination may be helpful (See also Section 20).
18.2.1. Inspection
Where is the mass located? A practical approach is to divide the abdomen into four quadrants (See Section 20.1). Starting from the principle that an abdominal mass originates from an organ, surface anatomy may suggest which one is enlarged. A mass seen in the left lower quadrant, for example, could be of colonic or ovarian origin but, unless there is situs inversus, one would not consider an appendiceal abscess. Does the mass move with respiration? In the upper abdomen a mobile intraabdominal mass will move downward with inspiration, while a more fixed organ (e.g. aorta, pancreas) or an abdominal wall mass (e.g. hematoma of the rectus muscle) will not.
18.2.2. Auscultation
Careful auscultation for bowel sounds, bruit or rub over an abdominal mass is part of the systematic approach.
18.2.3. Defining the Contour and Surface of the Mass
This is achieved by inspection, percussion and palpation. Is the organ air filled (e.g. stomach) or fluid-filled? Is it a well-defined mass (e.g. liver, spleen) or are its borders difficult to define (e.g. matted loops of small bowel)? Is the surface regular? An enlarged liver due to fatty infiltration may have a smooth surface. What is the consistency of the mass? Firm, hard or soft? Is it pulsatile to suggest an aortic aneurysm? In the absence of ascites, ballottement of an organ situated in either upper quadrant more likely identifies an enlarged kidney (more posterior structure) than hepatomegaly or splenomegaly.
18.3. Differential Diagnosis
The following suggests an approach to the differential diagnosis of an abdominal mass located in each quadrant:
18.3.1. Right Upper Quadrant
This location suggests liver, right kidney, gallbladder and, less commonly, a colon or gastroduodenal mass. A pancreatic mass is rarely palpable.
Liver: As a subdiaphragmatic organ, the liver moves downward with inspiration. This anterior organ has an easily palpable lower border, which permits assessment of its consistency. An enlarged left lobe can usually be felt in the epigastric area.
Right kidney: The kidney may protrude anteriorly when enlarged and be difficult to differentiate from a Riedel’s lobe of the liver. It may be balloted.
Gallbladder: This oval-shaped organ moves downward with inspiration and is usually smooth and regular.
Colon: Colon masses are deep and ill-defined, and do not move with respiration. High- pitched bowel sounds suggest obstruction.
18.3.2. Left Upper Quadrant
Location in the left upper quadrant suggests spleen or left kidney. Less commonly, a colonic (splenic flexure) or gastric mass can be felt. A pancreatic mass is rarely palpable.
Spleen: This anterior organ moves downward with inspiration. Since it has an oblique longitudinal axis, it extends toward the right lower quadrant when enlarged. It has a medial notch and the edge is sharp.
Left kidney: Its more posterior position and the presence of ballottement helps distinguish the left kidney from the spleen.
Colon, pancreas, stomach: It is practically impossible to differentiate masses in these organs by physical examination. The history helps but often one must resort to radiology or endoscopy.
18.3.3. Right Lower Quadrant
A mass in this area has its origin either in the lower GI tract (colon, distal small bowel, appendix) or in a pelvic structure (ovary, uterus, fallopian tube).
Lower GI tract: These deeper organs are usually ill-defined. Clinical context is important. Inflammatory bowel disease usually would be associated with pain on palpation but carcinoma of the cecum would be painless.
Pelvic organs: Bimanual palpation is the preferred method.
18.3.4. Left Lower Quadrant
As with a right lower quadrant mass, the differential diagnosis here is between lower GI (in this quadrant the sigmoid colon) and pelvic origin. Pelvic examination may help differentiate the two.
18.4. Approach to Diagnosis
To complete the assessment of an abdominal mass, one may choose among several different investigational tools. The use of specific tests depends on availability and on the organ studied. Generally, ultrasound is useful. This noninvasive, safe, cheap and widely available method identifies the mass and provides information on its origin and nature. Ultrasound may also be used to direct a biopsy. Other noninvasive modalities are CT scan and MRI. Hollow organs may be demonstrated radiographically through the use of contrast media (e.g., air contrast barium enema, upper GI series, intravenous pyelogram, endoscopic retrograde cholangiopancreatography [ERCP]). Sometimes, laparotomy or laparoscopy will be necessary to make the diagnosis.
19. Proctalgia Fugax / W.G. Thompson and N. Saloojee
19.1. Description
Proctalgia fugax is a sudden severe pain in the anus lasting several seconds or minutes and then disappearing completely.
19.2. Mechanism
The pathophysiology of proctalgia fugax is uncertain. Although some observations suggest a rectal motility disorder, the symptom appears more likely to result from spasm of the skeletal muscle of the pelvic floor (specifically, the puborectalis).
19.3. History and Physical Examination
Proctalgia fugax occurs in about 14% of adults and is somewhat more common in females than males. The pain may be excruciating, but since it is so short-lived patients seldom report it to their physician. In 90% of instances it lasts less than five minutes and in many cases less than a minute. About one third of patients suffer attacks following defecation. A small minority report attacks following sexual activity. There are no physical signs.
19.4. Differential Diagnosis
Perianal disease may cause pain but it usually accompanies, rather than follows, defecation. One should be particularly careful to exclude the presence of an anal fissure, which may be difficult to see on anal inspection. Pain originating from the coccyx may be accompanied by coccygeal tenderness both externally and from within the rectum. An acute attack of anal pain lasting several hours may indicate a thrombosed hemorrhoid.
Management
Beyond reassurance, there is no specific treatment.
20. Examination of the Abdomen / R.F. Bursey, J.M. Fardy, D.G. MacIntosh and N. Saloojee
Examination of the abdomen is an important component of the clinical assessment of anyone presenting with suspected disease of the gastrointestinal tract. As in all other parts of the examination, care must be taken to show respect and concern for the patient while ensuring an appropriate and thorough examination. While performing the examination it is useful to keep in mind the concepts of sensitivity and specificity. How confident can we be that a suspected physical finding is in fact present and has clinical significance? For example, how sensitive and specific is our bedside examination for hepatomegaly? What is the clinical significance of an epigastric bruit heard in a thin 20-year-old female versus a 55-year-old hypertensive, obese male? In the following sections we will describe an appropriate sequential examination of the abdomen and highlight some of the potential pitfalls of this process.
20.1. Inspection
Start from the usual position to the right side of the supine patient. The patient should not have a full bladder. Ensure that the abdomen is exposed from the costal margin to symphysis pubis. Positioning is important. The patient should keep his or her arms by the sides or folded across the chest. Ensure the patient does not have his or her legs crossed. When describing the location of an abnormality it is useful to divide the abdomen into four quadrants. Imagine a perpendicular line through the umbilicus from the xiphoid process to the symphysis pubis. A horizontal line through the umbilicus then allows the abdomen to be divided into 4 areas: the left upper, right upper, left lower and right lower quadrants (Figure 3). On occasion it may be helpful to divide the abdomen into 9 regions with the spaces marked by vertical lines through the left and right mid-clavicular lines and horizontal lines passing through the subcostal margins and anterior iliac crests (Figure 4).
The overall appearance of the abdomen can be described as scaphoid (markedly concave), protruberant, or obese. The location of any surgical scars noted. One should examine the skin for cutaneous lesions, vascular markings, dilated veins and striae. Note any pulsation that could indicate an aneurysm. Note the movement of the abdominal wall with respiration. Normally the abdominal wall will rise with inspiration. Occasionally organomegaly or a mass will be visible. It is helpful to look at the abdomen from the foot of the bed as well.
Figure 3. Division of the abdomen into four quadrants: the left upper quadrant, right upper quadrant, left lower quadrant and right lower quadrant.
Figure 4. Division of the abdomen into nine regions.
20.2. Auscultation
It is useful to auscultate the abdomen for bowel sounds and bruits prior to palpation or percussion. Palpation or percussion will stimulate peristalsis and may mask vascular bruits. When listening for vascular bruits it is useful to keep in mind Figure 4. Bruits are vascular sounds created by turbulent flow and may indicate partial arterial occlusion. Arterial bruits are usually heard only during systole and best heard with the diaphragm of the stethoscope, as they are high pitched. Listen for the aorta in the epigastrium. Renal bruits may be heard midway between the xiphoid process and the umbilicus, 2 cm away from the midline. Listen for iliac bruits halfway between the umbilicus and the inguinal ligament. One should listen over the inguinal ligament for femoral bruits as well.
About 20% of normal persons will have a vascular bruit, so that the auscultation of an abdominal bruit has to be placed within the clinical context.
A venous hum is a rare sound, best heard overlying the portal vein. This is found an area approximated by an ellipse between the umbilicus and the midclavicular line where it crosses the right subcostal margin. A venous hum can occur in portal venous hypertension of any cause. There are, however, no studies to suggest this is a helpful finding in routine examination.
Friction rubs are a rare sound indicating inflammation of the peritoneal surface of an organ. They are grating in quality and vary with respiration. They may occur over a liver tumour. However, even with careful auscultation of patients with known liver tumours, fewer than 10% are found to have a rub.
20.2.1. Bowel Sounds
Bowel sounds should be listened for prior to palpation or percussion, but the yield of this examination is low. The diaphragm of the stethoscope should be placed on the abdomen. Listening in one spot, such as the right lower quadrant, is generally sufficient since bowel sounds are transmitted widely through the abdomen. Rushes of very high pitched bowel sounds coinciding with crampy pain may indicate hyperperistalsis and acute small bowel obstruction. Complete absence of bowel sounds may indicate an ileus or peritonitis.
20.3. Palpation
Palpation of the abdomen should be done in an orderly sequence with the patient in the supine position. Light palpation should be done in all four quadrants, assessing for areas of potential tenderness. If the patient complains of pain, palpate that area last. With one hand, using the pads of the fingertips, palpate in a gentle, circular motion. If no areas of obvious tenderness are elicited, then deep palpation is performed. This is again done in all four quadrants, however use both hands. One hand is placed on the abdominal wall. The second hand is placed over top of the first. It is thought that using one hand for deep palpation may increase the risk of missing a mass. The accuracy of this has not been tested.
Involuntary guarding and rebound tenderness are signs of peritoneal inflammation (peritonitis). Guarding refers to contraction of abdominal wall muscles when the abdomen is palpated. Guarding is only important if muscle contraction is involuntary. Involuntary guarding occurs as a protective mechanism when peritonitis is present. Voluntary guarding occurs when a patient tenses abdominal wall muscles in response to that abdominal wall pressure. It is a meaningless finding that is commonly seen. Guarding may be localized (e.g uncomplicated appendicitis) or generalized throughout the abdomen (e.g. perforated appendicitis with diffuse contamination of the peritoneal cavity). In some instances of peritonitis, the muscles are in a state of continuous contraction. They are rigid or “board-like” even without palpation. In subtle situations, peritonitis is suggested by the triggering of pain in the area of suspected pathology (e.g., appendicitis) through palpation elsewhere on the abdominal wall, by having the patient cough or by gently shaking the pelvis.
Rebound tenderness, another sign of peritonitis, is elicited by deeply palpating the area of concern and then suddenly releasing the abdominal wall. Severe pain felt on release of the abdominal wall is rebound tenderness. This manoeuvre can be very distressing to the patient with peritonitis, so it is often not done. The sites for inguinal and femoral hernias should be specifically examined. Femoral pulses should be palpated.
The techniques of palpation of liver and spleen are discussed in Sections 20.5 and 20.6.
20.4. Percussion
Percussion of the abdomen will detect the presence of bowel gas. It is useful in defining organomegaly and the presence of free intra-abdominal fluid (ascites), as discussed below.
20.5. Examination of the Liver
First, inspect for a right upper quadrant mass. The examiner should look for stigmata of chronic liver disease (section 15.3). Next, palpate for the lower edge of the liver.
To palpate the liver edge, start in the right lower quadrant of the abdomen. The edge of an enlarged liver may be missed by starting too high in the abdomen. The patient is asked to breathe deeply and slowly, in order to bring the liver edge down to the examining fingertips of the right hand. The examiner moves the right hand in a cephalad direction about 2 cm with each expiration. If the edge is not felt, no further examination is required.
The liver, if palpable, is normally smooth and regular. Note any firmness, irregularity or nodularity to suggest an abnormal liver. Interobserver agreement about such characteristics is poor, even among experts. Note any tenderness. When the liver edge is palpable, trace the edge working laterally to medially. If the liver edge is palpated, the liver span should be measured by percussion. Start by percussing in the right midclavicular line in an area of tympany. Percuss in a cephalad direction in the right midclavicular line until an area of dullness is encountered. This is the lower border of the liver. Percuss for the upper border starting in the right midclavicular line in the third intercostal space. Move down one interspace at a time until the percussion note changes from resonant to dull. To confirm the change of percussion note strike the third and fourth fingers laid in adjacent interspaces. The note on the top finger should be resonant and on the lower dull. This is the upper border of the liver. Measure the distance between the upper and lower percussion edges in the mid-clavicular line. Percussion typically underestimates the true vertical span on the liver.
The scratch test has been used to find the lower liver margin. The diaphragm of the stethoscope is placed at the right costal margin in the midclavicular line. A finger moves up the abdomen in the mid-clavicular line, scratching gently and with consistent pressure. When the liver edge is reached, there is a sudden increase in the scratching sound heard through the stethoscope. In one comparative study the scratch test was not felt to offer any advantage over the techniques of palpation and percussion.
What is the significance of a palpable liver edge? One review suggested that a palpable liver is not necessarily enlarged or diseased. When clinical examination is compared to nuclear medicine scanning, about one-half of palpable livers are not enlarged. The inability to feel a liver edge does not rule out hepatomegaly, but does reduce its likelihood. What is the normal percussion span? Only one study has been done to establish the normal span. Castell examined 116 healthy subjects using firm percussion. The mean span in the mid-clavicular line was 7 cm in women and 10.5 cm in men (8±2 cm in women and 11±2 cm in men is considered to be normal).
The following nomograms were developed to predict estimated liver dullness in a normal population using firm percussion technique. Male liver dullness equals (0.032 x weight in pounds) + (0.183 x height in inches) – 7.86. The female liver dullness equals (0.027 x weight in pounds) + (0.22 x height in inches) – 10.75. The 95% confidence intervals were ±2.64 cm. Therefore a 5 ft. 10 in., 175 lb. male would have an estimated liver span of 10.2 cm (range 7.6– 12.8) and a 5 ft. 5 in., 130 lb. female would have an estimated liver span of 7.1 cm (4.5–9.7 cm) by this formula. These formulae are not used in day to day clinical practice.
Unlike the kidney in the left upper quadrant, the spleen moves downward and medially with inspiration, and does not have a notch.
20.6. Examination of the Spleen
The normal spleen is a curved, wedge-shaped organ located beneath the rib cage in the upper left quadrant. The spleen lies beneath the left tenth rib and normally weighs about 150 g. It is approximately 12 cm in length, 7 cm in width and 3 cm in thickness. The normal spleen usually cannot be palpated, but as it enlarges it descends below the rib cage and across the abdomen toward the right lower quadrant. An enlarged spleen may have a palpable notch along its medial edge. Examination of the spleen should begin with observation of the left upper quadrant for an obvious mass, though such a mass is quite uncommon.
The examiner should then proceed with percussion over the area of the spleen to look for evidence of dullness, implying splenic enlargement. The two most useful methods are percussion over Traube’s space and Castell’s sign. The surface markings for Traube’s space are the left sixth rib, the left midaxillary line and the left costal margin. An enlarged spleen may cause dullness over Traube’s space. Percussion should be carried out at one or more levels of Traube’s space from medial to lateral. This maneuver has a sensitivity and specificity between 60 and 70% for splenic enlargement. The sensitivity and specificity increases to approximately 80% in non-obese patients who are fasting.
Castell’s method involves percussion in the lowest intercostal space in the left anterior axillary line. In normal individuals this area is resonant on percussion and remains resonant on inspiration. In patients with mild splenic enlargement this area will be resonant on percussion and become dull on maximal inspiration. This method has a sensitivity and specificity of approximately 80% for detection of splenic enlargement and is helpful for detection of a minimally enlarged spleen that may not be palpable.
Palpation of the spleen should begin in the right lower quadrant and proceed toward the left upper quadrant in order to follow the path of splenic enlargement. Palpation should initially be carried out in the supine position with a bimanual technique using the left hand to gently lift the lowermost portion of the left rib cage anteriorly. The fingertips of the right hand are used to palpate gently for the spleen tip on inspiration. The hand is moved from the right lower quadrant, advancing toward the left upper quadrant. If the spleen is not palpated in the supine position, the patient should be moved into the right lateral decubitus position and again with bimanual technique the spleen tip should be sought using the fingertips of the right hand on inspiration. This technique has a sensitivity of about 70% and specificity of 90% for splenic enlargement.
20.7. Examination for Suspected Ascites
The presence of ascites, free fluid within the abdominal cavity, is always due to an underlying pathological process (see section 16). Potential causes include cirrhosis, severe right- sided heart failure, primary intra-abdominal malignancy, peritoneal metastases, chronic infection such as tuberculosis, and lymphatic obstruction. It is easy to identify large-volume ascites clinically, but the sensitivity of the examination techniques falls with lower volumes of fluid. Ultrasound, which can detect as little as 100 mL of free fluid, is the gold standard against which the clinical diagnostic maneuvers are compared.
An approach involves inspection for bulging flanks, palpation for the presence or absence of fluid waves, and percussion to demonstrate shifting dullness. Bulging flanks are suggestive of ascites since fluid sinks with gravity, while gas filled bowel loops float to the top. Adipose tissue in the flanks may be occasionally mistaken for free fluid.
To demonstrate a fluid wave it is necessary to enlist the aid of the patient or another individual. With the patient in the supine position, the examiner places one palm on the patient’s flank. The patient or an assistant places a hand on the mid-abdomen. This is to apply sufficient pressure to dampen any wave that may pass through adipose tissue in the anterior abdominal wall. The examiner then briskly taps the opposite flank. If fluid is present, a shock wave will be felt with the palpating hand. The sensitivity of this technique is approximately 50% but it has a specificity of greater than 80%.
To test for shifting dullness, percuss from resonance in the mid-abdomen to dullness in the flanks. The area of transition is then marked and the patient rolled to the opposite side. For example, if flank dullness is demonstrated on the left then the patient should be rolled onto the right side. One should allow approximately 30 seconds for the fluid to move between the mesentery and loops of bowel into the inferior portion of the abdomen. The previous area of dullness in the left flank should now be resonant. It does not matter which side one chooses to start with. In three separate studies shifting dullness had a sensitivity that ranged from 60–88% and a specificity that ranged from 56–90%. In one study involving six gastroenterologists and 50 hospitalized alcoholic patients, the overall agreement was 75% for the presence or absence of ascites and reached 95% among senior physicians (i.e. experienced). The absence of a fluid wave, shifting dullness or peripheral edema is also useful in ruling out the presence of ascites.
21. Oral-Cutaneous Manifestations of GI Disease / N. Saloojee
21.1. Description
A number of gastrointestinal disorders are associated with oral or cutaneous manifestations. Some of the more common of these are presented here.
21.2. Specific Disorders
- Glossiti Inflammation of the tongue which can occur in a number of conditions. Seen in nutritional deficiencies such as iron or B12 deficiency. The tongue is smooth and can be enlarged.
- Oral thr Candidiasis involving the oropharynx. When seen in association with dysphagia, the patient likely has esophageal candidiasis.
- Cutaneous manifestations of Inflammatory Bowel Disease (IBD). Aphthous ulcers may occur in the oral mucosa of patients with IBD. Generally these lesions follow the course of the disease. They are seen when the intestinal disease is activ
- Erythema Nodosum is a panniculitis or inflammation of subcutaneous fat that has multiple associations, one of which is IBD. Red, tender, nodular lesions occur on the anterior tibial area. These lesions follow the course of the intestinal disease.
- Pyoderma Gangrenosum is an ulcerating, tender lesion with a dusky borde It often occurs on the anterior tibial area or around a stoma site. Lesions can progress rapidly. Lesions sometimes follow the course of the intestinal disease, however not always.
- Hereditary Hemmorrhagic Telangiectasia (HHT) or Osler-Weber-Rendu Syndrome. This disorder is characterized by vascular lesions including telangiectasias and arteriovenous malformati Such lesions occur in the brain, lung and GI tract. Clinical manifestations include epistaxis and GI bleeding. Numerous telangiectasias occur on the lips.
- CRES This syndrome is an acronym for calcinosis, raynauds, esophageal dysfunction, sclerodactyly and telangiactasia. Patients experience dysphagia and reflux symptoms. Calcinosis is a deposition of calcium in the soft tissue, often around the elbows. Raynauds is a discolouration of fingers due to vasospasm that often results from exposure to cold. In sclerodactyly, the skin of the fingers becomes tightened and waxy. Telangiectasia occur on the chest and back.
- A number of polyposis syndromes have cutaneous manifestati Gardner’s syndrome is a form of Familial Adenomatous Polyposis, patients develop hundreds to thousands of colonic polyps at a young age. Colon cancer is inevitable without colectomy, generally by age 40. Many patients develop epidermoid cysts of the face, scalp and extremeties. Multiple unerupted supernumerary teeth may be seen.
- Peutz-Jeghers syndrome is characterized by hamartomatous polyps, mucocutaneous hyperpigmentation and an elevated risk of various cance
- Dermatitis Herpetiformis is a prurtitic, vesicular ra It may be seen on the scalp, shoulders, elbows, knees or buttocks. It is associated with celiac disease. The skin lesions usually respond to the introduction of a gluten free diet.
- Cutaneous disorders of liver disease. As noted previously, patients with liver disease may become jaundice In cirrhosis, palmar erythema, telangiactasia, and caput medusa (dilated periumbilical veins) may also be seen. Patients with hemochromatosis, a condition of iron overload, may develop a bronze discolouration of the skin. Xanthomas, deposits of yellowish, cholesterol rich material, develop on the trunk and face of patients with primary biliary cirrhosis.
 Oval Useful news from Azerbaijan and Caucasus
Oval Useful news from Azerbaijan and Caucasus